260 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Color couplers are compounds which produce little or no color when oxidized alone in the hair fiber, but which produce new colored species when used in the presence of primary intermediates. These color couplers include m-diamines, m~aminophenols, polyhydric phenols, etc. The most commonly used couplers are resorcinol (R) and 2,4 diaminoanisole (DAA), which when oxidized with p-phenylenediamine give green and blue colored products, respectively, along with many other minor products. When studying these dyes, it is important to ascertain the primary intermediates and couplers used in a particular dye, and secondly, the colored oxidation products formed from the dye precursors with the dye developer, i.e., hydrogen peroxide. To study these aspects, improved methods are needed for the separation and identification of oxidative hair dyes. Much work has been done in developing separation techniques to identify dye inter- mediates and other components present in oxidative hair dyes. These methods include the use of paper chromatography (PC) (1-6), gas chromatography (GC) (7-10), thin- layer chromatography (TLC) (11-19), and/or combinations of these chromatographic techniques (11,16), and other methods (20-23). In order to understand the mechanisms of the color-forming reaction, it is vital to identify the colored species produced during the hair dyeing process. Application of chromatography of the oxida- tion products of primary intermediates alone or in the presence of various couplers .: essential. Chromatography separation of the oxidation products of oxidative hair dyes has been reported (24-31), which emphasizes the importance of understanding the:.:::: redox reaction products of the dye intermediates. In the field of separation methods, chromatography occupies a rather unique pOsition,•)i and TLC provides the best answer to this problem in many cases. This paper describes' 7151 a comprehensive study of TLC of the oxidation products and their application to tire hair dye analysis. It presents the effect of 3 main factors of chromatographic•-:•!i:i! ' separations, i.e., solutes (nature and amount) sorbents (quality and nature, thicknesS?• ' and uniformity, activation and sotrage) and solvent (quality and nature, vapor saturaf!i!i! tion) of the separation of oxidative dyes. Also, an isolation procedure for the desired component of the oxidation mixture afte•)• their separation is shown. This paper should (a) serve as abackground of informatiø:n•'•'i!• for those who would be utilizing this technique for identifying oxidation dye and (b) establish variables of this analytical method to provide the best separation nique for the multicomponent dye product. EXPERIMENTAL AND DISCUSSIONS The use of TLC for oxidative dyes presents 2 specific problems: first is the stability, and second is the problem of closely related structures of the products (Tables I, II). As mentioned previously, 2 of the primary ingredients are matic diamines and polyhydric phenols. The chemical transformation in the react• system, once initiated, may continue indefinitely. Even the individual fractions iso are self-reactive and/or react with each other and with foreign agents such as air, rn ture, sunlight, proteins, etc. which are manifested by several types of reactions in state or in solution. ,':•i•
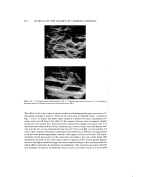
THIN-LAYER CHROMATOGRAPHY Table I RF Values of the Oxidation Products of p-Phenylenediamine and m-Methoxyphenol(a) 261 Band Color Relative Intensity RF X100 71!::!.' Redish Brown (origin) Strong 0 •ii:!:•:*'.'• Yellow Orange Strong 12.05 •,'•=':. Pink Strong 17.64 Purple Light 21.47 Yellow Strong 26.17 :-: ', Brown Strong 29.70 Yellow Medium 35.58 i!::.: Green Light 40.58 Orange Medium 41.76 :.:::•::•. .:. 1'1 mixlure with hydrogen peroxide oxidant and sodium carbonate base Chloroform' Ethylacetate: Methanol (6: 2: 2) solvent system. i • •.•:', '. ? •.::: •:::.:•There is a great advantage in working with these oxidative dyes. Since the products are •5 colored, it is easy to visualize the progress of the chromatography during development, ito obtain useful information on the presence or absence of certain components, and '. ii even to make a rough estimate of their relative abundance. Although, PDA yields a number of color components in its reaction with alkaline peroxide both alone and with the individual couplers, the dominant composite colors are a purplish-brown for PDA, a green for PDA-resorcinol, and a purplish-blue for PDA-diaminoanisole. The oxidative reaction of resorcinol and diaminoanisole alone or in combination produce negligible color compared with their coupling products with PDA. Self-coupling of PAP provides multicomponent products like other products, but gives a major yellow color. The binary coupling with resorcinol provides green color similar to that of PDA-resorcinol R, but with coupling with DAA the pre- dominant product is a vivid red with blues, greens and relatively few brown components either in primary, or in polymeric products. Chemical structures of the major colors are shown in Fig. 1. With prolonged dye development time, all the above compounds increasingly convert to polymeric compositions of brown components, having a very low mobility on thin layer plates.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)