278 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS by the fiber which quickly reduces with time. On further immersion of the fiber, the upward force on the fiber is reinstated (in fact it is slightly higher due to the additional buoyant force on the fiber). This pattern is observed during the immersion of each millimeter of the fiber up to a depth of 5 min. When the fiber is left in the water, the upward force slowly decreases, and in most cases, the direction of the force changes to the downward (positive) direction and reaches an approximately constant value. This value is referred to as the "equilibrium" wetting force we. All the results pertaining to the so-called "equilibrium" condition are designated by the subscript 'e'. The advancing wetting force is calculated from the 5 force readings obtained during the immersion of millimeter lengths of the fiber by linear regression analysis using eq. (4). The intercept is the advancing wetting force Wa. Results pertaining to this condition are designated by the subscript 'a'. The time-dependent change in the wetting force toward more positive values cannot be attributed to absorption of water into the fiber, because the amount absorbed is much too small to account for the difference between wa and we. To understand the time dependence of the wetting behavior of hair fibers illustrated in Fig. 1, glass, nylon, and polypropylene fibers were examined [9], and qualitatively all of them exhibited a similar behavior, i.e., we wa. In the case of glass and nylon fibers, both we and wa were positive, whereas, in the case of polypropylene both were negative. In the case of untreated hair, we was positive in most cases and wa was negative. Even a platinum wire exhibited the same behavior. However, the difference between the two wetting forces (we - wa) was much larger for hair and nylon fibers than for glass and polypropylene fibers and the platinum wire. This would suggest that the time-dependent increase in the wetting force occurring when the fiber is left in the water at a depth of 5 mm may at least partly be due to interaction of the liquid with the surface molecules of the solid in the case of hair and nylon, hydrogen bond breaking can occur. It is possible that the lower wetting force w•, observed when a new interface is established between the solid and the liquid, is partly due to the high advancing velocity (-15 ram/rain) and partly due to the lowering of the surface energy of the solid as a result of adsorption of molecules of water vapor during conditioning at 65 per cent RH. When such a condi- tioned solid is brought into contact with the liquid surface, adsorbed liquid molecules reorient at the solid-liquid interface reducing •'sL, thus increasing the wettability of the solid surface. This phenomena has been observed by Shafrin and Zisman [10] in the case of glass at various relative humidities. In the case of polymers that interact with the liquid, however, a further increase in wettability would be caused by relaxation of pol.ymer molecules in the surface regions and orientation of polar groups toward the liquid and nonpolar groups away from the liquid. This situation is strongly indicated in the wetting behavior of hair fiber against water. EFFECT OF WEATHERING AND MECHANICAL DAMAGE In the case of long hair fibers, the extent of weathering and mechanical damage is likely to be greater near the tip than near the root. Therefore, a 10-in. long hair fiber was cut into 6 sections and the wettability of each section was determined by immersing the end closest to the tip. The advancing and "equilibrium" contact angles calculated from measured wetting forces are reported in Table I. In this Table, A is the tip section (may not be the natural tip) and F is the root section. As can be seen, the tip end is more hy-
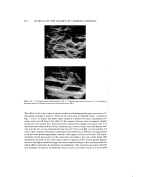
KERATIN FIBER SURFACE Table I Contact Angles (Calculated) of Water Against Hair Measured Along the Length of the Fiber Fiber section 0,,(degrees) 0,,(degrees) A 72 64 B 73 68 C 67 68 D 99 81 E 101 76 F 103 89 279 drophilic than the root end. This may be due to degradation of the protein by the ul- traviolet rays of the sun or by environmental factors that generate hydrophilic groups at the surface, or it may be due to loss of cuticle by mechanical damage. To establish the difference in the wetting behavior of the cortex and the cuticle, wet- tability of the tip (natural tips were chosen by viewing in the microscope) and the root ends of hair fibers were measured. Damaged tips with the cortex exposed yielded posi- tive wetting forces, while the root ends, with cuticle intact, gave negative wetting forces. Scanning electron micrographs of one of these fibers are shown in Fig. 2 along with the measured advancing wetting forces. EFFECT OF CHEMICAL OXIDATION AND REDUCTION Oxidation of hair samples was carried out with a 3 per cent solution of hydrogen peroxide adjusted to a pH of-10 with 0.1 N ammonium hydroxide. Single fibers mounted on hooks were immersed in this solution for 2 rain at a time followed by exhaustive rinsing with distilled water. Fibers were conditioned at 65 per cent RH and ?0øF prior to measurement. Successive treatments of 2-rain duration were carried out on the same fibers. Reduction was carried out in the same way using a 2.5 x 10 --• M so- lution of dithiothreitol, but the fibers were rinsed with deoxygenated distilled water. The increase in wetting expressed as work of adhesion (see equation (7)) caused by both oxidation and reduction are shown in Fig. 3 as a function of treatment time. As ex- pected, both oxidation and reduction increase the wettability of the surface. These increases are attributed to the generation of sulfonic acid groups in the case of oxida- tion and of thiol groups in the case of reduction, both of which are hydrophilic. The data presented here are inadequate for a comparison of the hydrophilic nature of the scission products and for the determination of the extent of disulfide cleavage in these 2 reactions. Wettability or work of adhesion are only indirect means of assessing the quantitative effects of these reactions. Oxidation of cystine by hydrogen peroxide is complicated by the reversible nature of several intermediate steps eventually leading to the formation ofcysteic acid [11]. It is possible that under these conditions of oxidation cleavage of peptide linkages may also occur to some extent, which may account for the discontinuous nature of the "oxidation" curve in Fig. 3 after a 6-rain oxidation time. Reaction of hydrogen peroxide with peptide bonds is known to occur in wool and silk at 60øC [12], and, though the reaction may not be extensive at room temperature, it may not be ruled out. It should be noted that wettability or work of adhesion is a measure of reactions occurring at the fiber surface only.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)