ANALYTICAL CHEMISTRY OF COSMETICS 409 sector spectrometers, has enabled smaller companies to acquire this instrumentation. The interfacing of both packed and capillary gas chromatographs with the mass spectrometer followed by computer data acquisition gives us a powerful tool for simultaneous separation and absolute structure elucidation. Recent technology has also interfaced HPLC to the mass spectrometer and this combination should prove valuable in separation and identification of nonvolatile mixtures. Both prism and grating infrared spectrophotometers have tradiationally been used for the characterization of cosmetic ingredients. Low cost Fourier transform infrared spectrometers are now available offering increased and constant resolution throughout the entire spectral range. Bench-top models can acquire spectra within seconds, and as with mass spectrometers, these systems are microprocessor and computer-assisted, allowing the chemist to search reference spectra for absolute identification. A new breed of hyphenated techniques such as GC-MS, LC-MS, GC-FTIR, LC-FTIR, and GC-FTIR-MS are finding increased application in chemical analysis. These systems contain both non-destructive and destructive analysis in tandem allowing the chemist to obtain several forms of spectroscopic information by performing a single experi- ment. Just recently the development of automated analysis system incorporating •3C-NMR, GC-MS and a computer has been reported on in detail (2). SURFACTANTS Surfactants are one of the most widely used classes of cosmetic raw materials. Comprehensive reviews are available to the reader (3,4) defined as compounds containing both a "hydrophilic" and "hydrophobic" group, the molecules can locate between the interface of an organic and aqueous phase. The materials are used as components in shampoos, conditioners, lotions, and creams. The artionic surfactants which are a large portion of this class of compounds are usually sulfate esters of long chain fatty alcohols having the general formula: CH3(CH2)n--OSO3-Na + where n = 11, 13, 15, or 17. Since most are derived from natural sources, they are usually mixtures of homologs, and their effectiveness and physical properties depend markedly on the alkyl chain length distribution. Initial analytical approaches have focused on the anionic portion of the molecule, and the greatest number of methods reported in the literature involve the use of dyes as a complexing agent. Thus, early literature reported that colored, water-insoluble salts were formed between methylene blue and anionic surfactants and that the reaction products were soluble in organic solvents such as chloroform (5). This observation was developed into a colorimetric determination with the sensitivity of the method being 20 ppm of surfactant (6,7) and is the basis for the anionic titration method currently and frequently used in the cosmetic industry. The determination of anionic surfactants using polarography has also been explored, but the reduction in height of the half-wave potential of methylene blue, buffered at pH 4.5, by the addition of anionic surfactants, was shown to be non-specific (8). The use of infrared spectroscopy for the determination and identification of surfactants has grown over the years. The major drawback is in the case of mixtures of several
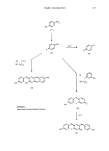
410 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS components the spectrums become complex and diffuse, and it becomes increasingly difficult to recognize anything other than particular functional groups. Consequently, the infrared spectrum of a commercial surfactant may bear only a slight resemblance to its isolated major component. Useful information can be obtained if water is carefully removed from the sample, since the presence of even traces of moisture can mask surfactant absorptions in the 3400-3000 cm -•, 1700-1500 cm -•, and 600 cm -• region (all associated with H20). The more common assignments of functional groups associated with anionic surfactants are the hydroxyl-OH stretching frequency occurring between 3400-2400 cm-L This band is usually complex and broad. The CH- stretching frequency is usually superimposed on the low side of the broad OH- band. Absorption arising from the CH- stretching of the methylene (CH2) and methyl (CH3) groups on the carbon chain occurs between 2950-2750 cm -•. Unsaturation can be observed at 3020 cm-•, and its intensity relative to the methylene antisymmetric stretch at 2920 cm-• can give an indication as to the degree of unsaturation (9-14). More recently, gas chromatography has played an increasingly important role in the characterization of anionic surfactants, the best known being sodium dodecyl sulfate. This material can be easily hydrolyzed in acid media to yield the original fatty alcohol. It has been shown that about 98% of sodium lauryl sulfate is hydrolyzed in 1N HCI at 100 ø in seven hours (15). The hydrophobic oil is extracted from the reaction solution with suitable solvents, then gas chromatographed with or without pretreatment. Mixtures of fatty alcohols have also been separated by forming the acetates (16). Further work has been reported in the literature on the analysis of alkyl sulfates using on-line pyrolysis gas chromatography with and without reagents (17). At 650øC pyrolysis gas chromatography of commercial sodium lauryl sulfate gave peaks for the C•2 and C•4 fatty alcohols and the C•2 and C•4 ce-olefins. On-line P205 pyrolysis gas chromatography gave peaks due only to a mixture of olefins. The formation of the alkyl alcohols was not observed. A second class of anionic surfactants used widely in cosmetics are the ethoxylated ether sulfates of fatty alcohols, obtained by reacting the alcohol with ethylene oxide followed by sulfation. The general formula for these compounds is: CH3(CH2).O(CH2--CH2-O)mSO3-Na + where n = 11, 13, 15, 17 and m = 1-20. Naturally occurring sources usually contain the C•0, C14, and C16 alcohols in varying amounts. If one ethoxylates lauryl alcohol to an average of 2 ethylene oxide (EO) units, the number of reaction products obtained is considerable. Not only is the 2EO derivative present, but the homologs of the 1, 3, 4, 5, and 6EO derivatives are also formed. Impurities of the C•0, C•4, and C•6 alcohols along with their homologous EO derivatives are also present. Since cosmetic products are delicately balanced formulas, differences in the homologous ratio can affect both stability and performance of the product. This has generated research into the analysis and distribution of the homologous series contained in this class of surfactants. As with the alkyl sulfates, the alkyl ether sulfates can be hydrolyzed back to the ethoxylated alcohols. Gas chromatography of the hydrolyzed mixture on the porous polymer tenex or the liquid phase OV-17 has been used to separate the underivitized C•2EO oligomers with up to 8 ethylene oxide units, while the trimethylsilyl ether of the C•2 alcohol with up to 16EO units have been separated on Dow Corning high vacuum grease as the liquid phase (18).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)