ANALYTICAL CHEMISTRY OF COSMETICS 411 Researchers have found better resolution with capillary GC by converting the hydroxyl group to the methyl ether. The methylation was carried out with methyliodide and dimethylsulfonyl anion (19,29). An OV-101 glass capillary column was used for the separation, and a commercial C12-C14 alcohol ethoxylated with an average of 3 moles of ethylene oxide gave eleven peaks under these conditions. This method is quantitative and therefore suitable for fast routine quality control (21). Mass spectroscopy has been used for absolute identification of the separated peaks obtained from the hydrolysis of ethoxylated sodium alkyl ether sulfates. Traditional electron impact mass spectrometry on these compounds does not show a molecular ion. However, we have demonstrated in our laboratory that isobutane chemical ionization mass spectroscopy can be used to obtain the molecular weights of the separated components. Under these conditions, the alcohols show a strong (M - 1) ion whereas the ethoxylated alchols show a strong (M + 1) ion (22). We have also demonstrated (23) that silylation of the hydrolyzed surfactant will yield electron impact spectra that are suitable for structure elucidation. There is no doubt that mass spectroscopy yields the most complete picture of the chemical distribution of this class of surfactant. Non-ionic surfactants consist of ethylene oxide (or propylene oxide) capped with a hydroxyl group on one end and a long chain alkoxy group or alkyl phenol group at the other end. Examples of this class are alkyl ether alcohols, or the ethoxylated octyl and nonylphenols. These surfactants are very amenable to analysis by gas chromatography and other advanced analytical techniques (24). An excellent paper has been published on the applications of mass spectroscopy to the analysis of non-ionic surfactants, using electron impact solid probe mass spectroscopy. The authors characterized the most common ions obtained from ethoxylated alcohols, octyl and nonylphenols, fatty amines and ethoxylated fatty alcohols (25). The degree of ethoxylation in ethoxylated non-ionic surfactants is an important parameter which influences product performance and stability. Nuclear magnetic resonance spectroscopy has been used for the determination of this parameter (26-29). The technique is based on the observation that a proton placed in an external magnetic field can be aligned with the external field. Energy can be absorbed by the proton and flipped so that the alignment is against the external field. This is the less stable situation. Upon realignment with the external field, energy is released. These spectra are usually obtained as a plot of signal intensity vs. change in strength of applied magnetic field. The CH 3 protons of trimethylsilane (TMS) are used as an internal standard and are arbitrarily set at 0 ppm or 10% Since TMS contains 9 equivalent protons, a strong singlet is obtained. The methylene protons associated with the ethylene oxide chain are shifted downfield to 6.0-6.5% while the methylene protons from the alkyl chain occur at 8.0-8.5% By silylating the terminal alcohol with a strong silylating agent (i.e., Bis-(trimethylsilyl) trifluoroacetamide), one can ratio the integrated areas in the NMR for the 9 methyl protons of the derivitizing agent, with either the integrated areas for the protons from the ethylene oxide chain or the protons from the alkyl chain. Average alkyl chain length or average degree of ethoxylation can then be calculated. If the surfactant is octyl or nonylphenol, the integrated area corresponding to the four aromatic protons, which are shifted downfield to 2.0-3.03', can also be used for the ratioing. This technique has also been applied to propylene oxide adducts of alkyl phenols, fatty alcohols, and ethoxylated mercaptans.
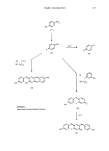
412 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS A substantial improvement in the resolution of the proton magnetic resonance spectra can be obtained by the addition of so-called "shift reagents" (30-32). Typical shift reagents are complexes of the rare earth metals, europium and praseodymium with ligands such as 2,2,6,6-tetramethyl-3,5-heptanedione or 1,1,1,2,2,3,3-heptafluoro-7,7- dimethyl-4,6-octanedione. These are available commercially as standard reagents for NMR. They induce large changes in the chemical shifts of the NMR spectra of compounds which possess functional groups with free electron pairs capable of forming a coordinate bond with europium or praseodynium ions: for example, alcohols. The improved resolution results in the largest shift occurring for the protons closest to the coordination site. The first application of shift reagents to this field was the structural study of the ortho and para-alkyl isomers of phenol polyglycol ethers. The spectrum of the ortho isomers showed greater shift when complexed than the para isomers (33). Several researchers have reported on the use of HPLC for the characterization of surfactants (34,35). Studies have been done on esters of polyoxyethylene monododecyl ether prepared by reaction with 3,5-dinitrobenzoylchloride followed by HPLC of the ethoxylated species (36). Compounds containing more than 12EO units were difficult to chromatograph. Ethoxylated alkylphenols have also been separated using this esterification technique. The 3,5-dinitrobenzoylchloride was allowed to react for 30 minutes at 65øC in 20 ml of pyridine, followed by extraction in THF and separation by HPLC. A Lichrosorb RP-5, 5 um size column (Merck, Darmstadt, G.F.R.) with a mobile phase of acetonitrile/water (6:4) was used. The column was thermostatted at 50øC. Work has been reported on the separation of surfactant homologs by HPLC using an ODS/silica column (37). The authors were successful in obtaining separation of nine typical surfactants. Water/methanol adjusted to pH 2.2 with phosphoric acid and water/methanol containing 0.4M N•CI were used as the mobile phase. Further work on the separation of EO oligomers by HPLC has been done with isocratic and gradient elution (38,39). a-Olefinsulfonates (AOS) are one of the newest class of surfactant raw materials being used in cosmetic products. The surfactant is manufactured by sulfation of a-olefins obtained from either cracking of or polymerization of ethylene. The surfactants are thus mixtures of isomers and homologs having a wide distribution of chain lengths (C•0 to C20). Desulfonation of AOS has not been successful. However, successful GC analyses have been obtained after hydrogenation followed by the formation of the volatile sulfonyl chloride. The hydrogenation was followed by IR using the disappear- ance of the 965 cm -• band, and the GC analysis of the final mixture of the sulfonyl chlorides was performed using a 3% SE-30 column (40,41). The term "cationic surfactant" refers to compounds containing at least one hydro- phobic long chain alkyl group and a positively charged nitrogen. Generally referred to as "Quats," these materials are incorporated into cosmetic hair formulations imparting manageability and anti-static properties. Because of their inherent bacteriostatic properties, these compounds are also used as sanitizing agents, antiseptic agents, germicides, and fungicides. Temperature-programmed gas chromatography has been the general technique of choice for qualitative identification. The technique is hard to apply to quaternary ammonium salts without first fragmenting the compound into more volatile species. Attempts have been made to degrade certain quaternary ammonium compounds
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)