ANALYTICAL CHEMISTRY OF COSMETICS 415 retention times allowed peak-height or peak-area measurements to be used for quantitation in the range of 10 pmol to 25 nmols. Pre-column conversion of the amino acids to their damsyl derivative followed by HPLC also has given good separation of all 20 common amino acids (57). Gas chromatography coupled with flame photometric detection is used for the analysis of sulfur-containing amino acids (58). High pressure (performance) liquid chromatography of proteins is becoming more prevalent in the literature, with the experimentation focusing on various support phases and buffer systems. For example, peptides varying in size from di- to decapeptide have been separated on phenyl-corasil, Poragel PN, and Poragel PS using reverse phase conditions with acetonitrile-water (59) as the mobile phase. Researchers have modified this method with the addition of phosphoric acid to the mobile phase (60,61). The analysis of proteins has also been reported using gel-permeation chromatography (62) and nuclear magnetic resonance spectroscopy (63). In the case of gel permeation chromatography, the chemist is using the separation technique of HPLC to obtain molecular weight distribution (64) rather than identifica- tion of the fraction separated. Cationic polymers used in hair fixatives can be analysed using gel-permeation chromatography to obtain an average molecular weight. Polymer chain length is no doubt related to product performance therefore, improved methods of analysis using gel permeation chromatography for charged polymers should be forthcoming. TRACE CONTAMINANTS Trace analysis of cosmetic raw materials is one of emerging concern for the cosmetic analytical chemist. During the past several years, the regulatory climate has involved the cosmetic industry in three major areas: the analysis of nitrosamines, the analysis of dioxane, and the analysis of specific trace contaminants in raw material dyes used in cosmetic finished products. The analytical methodology used to determine the level of contaminants of concern will be discussed. For the past five years, the Cosmetic, Toiletry and Fragrance Association (CTFA) Nitrosamine Task Force has been studying trace level contamination of cosmetic raw materials, with their major focus the determination of N-nitrosodiethanolamine (NDEIA). Since NDEIA is a polar-nitrosamine, its determination stands apart from the general methodology for the analysis of nitrosamines. Volatile nitrosamines have been detected by gas chromatography employing either a nitrogen specific detector or a thermal energy analyzer (TEA) (65,66). Initial research which found trace levels of NDE1A in cosmetic products used a TEA (67). The instrument contains a pyrolyric oven which cleaves the weak N=N=O bond to produce NO. An inert gas is used to sweep the NO into an ozonator which then forms activated NO 2. When the NO2 decays to the ground state, the emitted energy is detected. A gas chromatograph is used at the front end of the pyrolysis oven for the initial separation. This instrument for the most part is nitrosamine specific and is currently the leading analytical technique used to meet government standards for volatile nitrosamines. The cosmetic chemists' problem is more difficult, since NDEIA is polar and appears sometimes in trace quantity in complex mixtures. The CTFA has developed methodol-
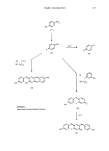
416 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ogy which uses a thermal energy analyzer for the analysis of seven classes of cosmetic raw materials (68): ethanolamines, ethanolamides, monoethanolamine salts, trietha- nolamine salts, amphoteric compounds, quaternary ammonium compounds, and morpholine. NDEIA and other polar nitrosamines are very amenable to separation by reverse phase HPLC using either water or water/alcohol mobile phases (69). The water/alcohol mobile phase provides good solubility for the raw material, eliminating the need to perform multiple isolation steps prior to analysis. Quantitation of the nitrosamine is by UV detection. Methods are in the literature for the analysis of NDEIA in ethanol- amines, alkanoladines, and cosmetic products (70-74). Likewise, polography and conductivity detectors have been used for the analysis of NDoe1A (75,76). Since the initial findings of NDoe1A in cosmetics, other nitrosamines have also been shown to be present in trace quantities, and methodology has been developed for each nitrosamine (77). These have all been polar compounds, and both water soluble and oil soluble nitrosamines have been studied. Some methods are available for total nitrosamine in cosmetic raw materials and finished products however, these are generally not rapid and certainly not specific (78). By far the most absolute method for nitrosamine detection is mass spectrometry and, when possible, should be used to confirm the presence of the nitrosamine of interest. Researchers have reported the determination of NDEIA by first forming its disilyl derivative followed by GC/MS analysis (79). The second half of the nitrosamine problem, that of nitrite analysis, has also been explored. The standard colorimetric test developed by Greiss (80) and its subsequent modifications (70,81) can determine low ppb levels of nitrite, while derivative formation followed by fluorescent spectroscopy can measure in the picogram region (82). The CTFA has published nitrite methodology which is specific for cosmetic raw materials (83). More recently, trace analysis for 1,4-dioxane has become significant for the cosmetic analytical chemist. 1,4-dioxane can be present, at trace levels, in some types of ethylene oxide condensates, and this broad class of compounds is widely used in both the food and cosmetic industry. Presently, the "Birkel procedure" (84), which consists of vacuum distillation of a sample followed by gas chromatographic analysis, is the accepted validated procedure. The total analysis time per sample is 2-3 hours with a 0.5 ppm limit of detection. Several other methods have been generated through the CTFA (85). Samples of widely used cosmetic ingredients (i.e., sodium laureth sulfate, Polysorbate 60, and PEG-8) were chosen for study. A total of seven generally different analytical techniques were employed, including the Birkel and modified Birkel procedures. Other methods generated used GC/MS with perdeuterotoluene as an internal standard (86), purge and trap procedures followed by GC, direct OC injection, headspace GC, and atmospheric azeotropic distillation followed by GC (87,88). The CTFA study showed that these alternate procedures yielded results comparable to the Birkel method, except for the purge and trap technique. It was felt that with some additional methods development work, the purge and trap technique could also be improved to the point where it too would be satisfactory. The last couple of years has seen an increased concern within both industry and government in establishing the safety of the dyes and colors used in cosmetic products.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)