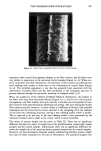
110 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS in water) by adding the 10 IxL of the agent onto the exposed skin area (0.32 cm 2) and conducting a TEWL rate-vs-time study. A skin-conditioning study was also done. Hamster ear skin was treated in vivo with daily applications (10 Ixl/ear) of mineral oil for two weeks. Animals were then sacrificed and TEWL determined for the previously treated and untreated contralateral ear skins. APPARATUS PREPARATION Syrian golden hamsters were sacrificed by means of an intraperitoneal injection of so- dium pentobarbital (65 mg per animal). The ears were removed by incision at the base with the aid of surgical scissors. The dorsal ear skin was gently pulled away from the supporting cartilage, starting at the base and extending distally. The cartilage was gently scraped off with a scalpel from the ventral side. A 10-mm diameter dermal punch was taken from the center of the stripped skin sample. A photograph of the teflon cell used for in vitro TEWL studies is shown in Figure 1. The cell was equili- brated to constant temperature at 32øC using heated water pumped through an alu- minum holding block from a 35øC water bath or from a temperature-controlled incu- bator. The 10-mm punch biopsy of the skin was placed ventral or hairy side up on the lip or edge of the donor compartment and the top holder was screwed tightly into place. The inside section of the top was free to rotate so that the top could be secured without twisting the skin. The exposed area of the skin was approximately 0.32 cm 2. With the membrane tightly fitted in place, one side of the side-arm opening was closed with a petrolatum plug. The donor compartment of the experimental cell (section un- derneath skin) was filled from the opposite side with approximately 150 IxL of 100 IxCi/ml solution (2.2 X 108 dpm/ml) containing tritiated water (HTO). This side was also closed with a petrolatum plug. A yellow Eppendorf pipet tip with a 5.0-mm section cut off from the bottom and, subsequently, attached into a 10-mm length section of ¬" O.D., Vs" I.D., V•6" wall Tygon tubing served as the receptor compart- ment. The Tygon tubing end was attached to the screwed top portion of the diffusion cell (see Figure 1). The receptor compartment was filled with approximately 0.24 g of granular, anhydrous calcium chloride, and the top covered with Parafilm. The cell was returned to the heated aluminum holding block or the incubator, and the desiccant was removed or exchanged according to the time specified. The desiccant was placed in a 20-mL scintillation vial and dissolved in 2 ml of purified water. A 500-uL aliquot sample was then placed into a new vial, dissolved in 10 mL scintillation cocktail (Aquasol-2, New England Nuclear, Boston MA), and counted for tritium content for one minute in a Model SL400 Intertechnique scintillation counter. A known volume of HTO was dissolved in water and the counts per minute (cpm) determined by scintillation counting. This was designated as the reference standard. A background count was determined for a volume of water and subsequently subtracted from the count for the reference standard. Both the reference and the blank standard had the same concentration of dissolved calcium chloride as the sample. This was to eliminate erroneous values due to quenching. The cpm was determined for the known volume of HTO and divided by density to determine the cpm/weight. An HTO count value was determined for sample and divided by the reference value, the time, and membrane area to determine the TEWL in Ixg/cm2/hr. Six replicates were prepared.
METHOD FOR TRANSEPIDERMAL WATER LOSS 111 A. Receptor compartment B. Donor compartment C. Parafilm seal D. Receptor compartment filled with calcium chloride. E. "Screw-in" attachment functions to secure skin and hold receptor cell. F. Skin membrane placed upon small well of water which functions as donor compartment. Figure I. In vitro TEWL apparatus, representing a modification of Bronaugh's flow-through diffusion cell.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)