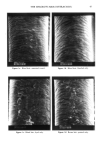
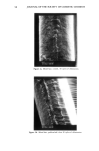
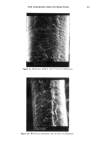
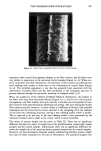
REACTION OF DEHYDROACETIC ACID AND FORMALDEHYDE 89 I. 280 1.02o 0.768 0.512 0.256 4 8 min. O. 16 O. 32 O. 48 O. 64 O. 80 Formaldehyde mg/ml Figure 2. Chromatographic pattern of a DHA.Na/formaldehyde water solution five weeks after storage and DHA.Na behavior in dependence on formaldehyde concentration. the above-reported data and from comparison with simple calculations of chemical shift, based on the usual additivity rules, we suggest the structure 3,7-dimethyl- 1H, 9H, 10H-dipyrano[4,3-b: 3 ', 4'-e]pyran- 1,9-dione (7). The 70 eV E1 mass spectrum of compound 1 is shown in Figure 3, while the related 1 OO- 246 Rel. Ab. 80- 60- 40- 20- 149 I 175 69 85 J 161 I ß J,,IL,,i,,,J .... , ..... I,,...,,, ..... ,,, ........ ,,, .... .•.,. ,...•L .... ,,i .... I, I i I 50 100 150 203 217 ,, ,, • ,, I,,i • Ii 200 250 m/z Figure 3. 70 eV EI mass spectrum of compound 1.
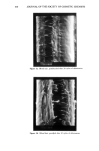
90 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 10 8 6 4 2 0 • Figure 4. •H-NMR spectrum of compound 1. fragmentation pattern is reported in Scheme II. Also in this case a good relationship between the proposed structure and the El-induced fragmentation pattern has been found. Most of the total ion current is retained by the molecular ions at m/z 246, which give rise to the base peak of the mass spectrum. This behavior is in agreement with the aromatic-like proposed structure. The primary fragmentation pathway consists in methyl loss, leading to [C12H705] + ions at m/z 231 and to CO and CHO' losses. These last two fragmentation pathways result quite typically in El mass spectrometry of qui- nones and pyranones (8). For the ions at m/z 218, the formation of a substituted furan 2 , +. rearr. ' • H3C-.•.•.•' O • m/z 149 (33) O •• 0 0 0 0 H •C13H1oO• +' • ........ rearrange7t M+',m/z 246 (1OO) m/z 203 (68) •C12H904] + m/z 217 (20) Scheme II • C9H503] + m/z 161 (9) •C 12H705] + m/z 231 (5) II 0 +' +.
OCH [C12H1oO4 ] - 3 m/z 218 (10) C oH70 3 m/z 175 (18)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)