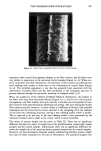
152 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS FormuLa # .• ß 28 1 .•4 ß 1o o.•5 [] 2• 0.88 o 1 0.06 ß t7 0.57 A 2 0.19 (•: 0i[/Water Phase-VoLume Ratia) I 4 8 24 28 32 48 52 56 72 Time, hours Figure 5. In vitro human skin permeation profiles of the selected hydrocortisone 17-valerate 0.2% emul- sions with •b ranging from 0.19 to 1.34. The bar indicates the standard error of the means (n = 3). higher than that of formula #2 (qb = 0.19). Figure 8 demonstrates the relationship of the permeability constants of formulas with a constant phase-volume ratio (qb = 1.34) but different % levels of petrolatum. A positive linear trend suggested that the perme- ability constants of HCV increase as the concentration of petrolatum increases. This was not the case for mineral oil, where the permeability constants of HCV did not increase as the concentration of mineral oil increased. Formulas # 1 and #36, the two extremes, both showed low permeability rates as compared to other formulas. The drying effect due to the high volatility was considered to result in the low permeability rate of formula # 1 (2). High viscosity with low driving force in the high petrolatum vehicle, which might cause the HCV to have a tendency to stay in the hydrophobic phase, was presumably the cause of the low permeability of formula #36. IN VIVO VASOCONSTRICTOR ACTIVITY DETERMINATION All the experimental results from the above studies suggested that formula #28, which contains 28.9% water, 40% petrolatum, and 10% mineral oil, is the most desirable
EMULSION VEHICLES AND VASOCONSTRICTOR ACTIVITY 153 4 % Formule # Petrolatum [] 28 40 o $t 50 & t9 20 [] 24 50 + 6 0 ß t3 t0 zx 36 70 0 4 8 2:4 2:8:32: 48 52: 56 72: Time, hours Figure 6. In vitro human skin permeation profiles of the selected hydrocortisone 17-valerate 0.2% emul- sions with 4) = 1.34. The bar indicates the standard error of the means (n = 3). choice for hydrocortisone 17-valerate. Formula #28 was then included in the compara- tive efficacy and safety evaluations against some marketed corticosteroid products. The degree of vasoconstriction induced by HCV 0.2% in formula #28 and a marketed cream, relative to that of other corticosteroid products, was evaluated by Sefton et al. (1) as shown in Table V. The results indicated that 0.2% HCV in both formula #28 and cream is associated with equal or greater vasoconstrictor responses than are other corti- costeroid preparations of intermediate or moderate potency corticosteroids, including formulations of triamcinolone acetonide 0.1% cream, fluocinonide 0.05% cream and ointment, and betamethasone 17-valerate 0.1% cream. It was also noted that formula #28 induces statistically greater vasoconstriction (by Duncan's procedure) than does HCV cream at 0.2% level. In order to examine the relationship between vasoconstrictor activity and skin perme- ation rate, an additional in vitro skin permeation study on the marketed HCV 0.2% cream was performed. According to the formulation information (7,8), the HCV 0.2% cream has a phase-volume ratio of 0.61. The resulting data from the skin permeation
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)