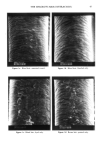
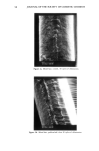
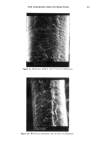
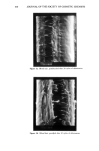
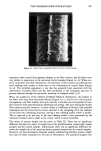
REACTION OF DEHYDROACETIC ACID AND FORMALDEHYDE 91 16.60 10 8 6 4 2 0 • Figure 5. •H-NMR spectrum of DHA. ring can be invoked, due to the higher stability of such a polycyclic structure. Analo- gously, for the ions at m/z 217 the formation of a pyranyl cation can be proposed. Finally, the cleavages 1 and 2 reported in Scheme II, of the polycyclic skeleton lead to ionic species [C9H503] + (m/z 161) and [C8H503] + (m/z 149) respectively, while the primary CH3CO loss leads to [CllH704] + ions. Having determined the structure of the reaction product of DHA.Na and formaldehyde in aqueous solutions, we thought it of interest to test its presence in cosmetic formula- tions containing DHA.Na and Bronopol © or Germall 115 ©. HPLC runs of a standard solution of compound 1 and of cosmetic emulsions (3 months stored at room temperature) preserved with Prevan-Bronopol © and Prevan-Germall 115 © are reported in Figure 6. The cosmetic samples were diluted 1:10 with THF/H20 (9/1) before HPLC analysis (9). In their chromatographic patterns there clearly appears a well-marked peak presenting the same k' = 1.76 of compound 1. In order to confirm its structure we collected the corresponding peak fractions that had undergone mass spectrometry analysis. The spectra, in accordance with the standard, gave unambiguous evidence of the reaction between DHA.Na and CH20 released by preservatives. Other ingredients of the cos- metic formulation did not affect the formation of 3,7-dimethyl-lH,9H,10H-di- pyrano[4,3-b: 3', 4' -e]pyran- 1,9-dione. CONCLUSIONS An interaction product between DHA.Na and formaldehyde released from other preser- vatives has been demonstrated by HPLC methods using aqueous solutions or cosmetic formulations. The structure of this unexpected product appears to be: 3,7-dimethyl- 1H,9H,10H-dipyrano[4,3-b:3',4'-e]pyran-l,9-dione, by elemental analysis, •H- NMR, mass spectrometry and X-ray analysis. In contrast to what has been suggested in the past, the degradation product exhibits a
92 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS o o O 2 4 6 min. O 2 4 o o 6 min. o 0 2 4 6 min. Reference sample cosmetic emulsion cosmetic emulsion preserved with Prevan preserved with Prevan and Germall 115 and BronoDol Figure 6. HPLC runs of cosmetic emulsions preserved with DHA.Na-Bronopol © and DHA.Na-Germall 115 © after dilution with THF/H20 (see method 2). molecular weight higher than DHA, due to the reaction of two molecules of DHA with formaldehyde. ACKNOWLEDGMENT This work was supported by a grant from the Italian C.N.R. Special Project on Fine Chemicals. REFERENCES (1) A. Bettero, A. Semenzato, G. Aversa, and C. A. Benassi, Imidazolidinilurea e formaldeide nei pro- dotti cosmetici, Chimica Oggi, 11, 29-32 (1985). (2) A. Bettero, F. Galiano, S. Daolio, and C. A. Benassi, The characterization of isothiazolinone pre- servatives in cosmetics, J. Pharm. Biotaed. Anal., 3, 581-587 (1985). (3) A. Bettero, A. Semenzato, and C. A. Benassi, Preservatives in cosmetics. Developments on direct characterization, Proc. 14 •h IFSCC, Barcelona, 16-19 September 1986, Vol. I, 187-194. (4) C. A. Benassi, A. Semenzato, M. Lucchiari, and A. Bettero, Dehydroacetic acid sodium salt stability in cosmetic preservative mixtures. Int. J. Cosmet. Sci. 1988 (in press). (5) C. A. Benassi, A. Bettero, A. Semenzato, and R. Cerini, Correlazione tra analisi chimica e previ- sione di stabillth microbiologica di un prodotto cosinerico. Proc. VII Congresso Naz. Div. Chim. Farm. Soc. Chim. It., Pisa, 22-26 Settembre 1987. (6) A. P. Bruins, K. R. Jenning, and S. Evans, The observation of metastable transitions in a double-fo- cussing mass spectrometer using a linked scan of the electric sector and magnetic sector fields, Int. J. Mass Spectrom. Ion Phys., 26, 395-404 (1978). (7) M. Moreno-Mafias, J. Ribas, and A. Virgili: The 3,6,9-trioxanthracene and 3,6-dioxa-9-thianthra- cene ring systems by cyclization of 1,1-bis[4-hydroxy-6-methyl-2-oxo-2H-pyran-3yl] alkanes, Syn- thesis, 3, 699-701 (1985). (8) Q. N. Porter, Mass Spectrometry of Heterocyclic Compounds (Wiley, New York, 1985), pp. 198-211. (9) A. Bettero, B. Casetta, F. Galiano, E. Ragazzi, and C. A. Benassi, Rheological and spectroscopic behaviour of cosmetic products. Application to sulphur analysis. Fresenius Z. Anal. Chem., 318, 525-528 (1984).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)