144 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table III Rating Scale of Cosmetic Acceptance Evaluation Rating Chara.c- .• cale teristics "• 1 2 3 4 5 Color Pure white Off-white Yellowish Yellow Brownish Appearance Very shiny Shiny Slightly Dull Unctuous shiny Odor Odorless Faint odor Faintly Unpleasant Extremely unpleasant unpleasant Homogeneity Very smooth Smooth Very slightly Slightly Non-uniform non-uniform non-uniform Texture Very soft Soft Slightly Stiff Vey stiff/ stiff hard Spreadability Very easy to Easy to Slightly Difficult Very difficult spread (no spread difficult to to spread to spread drag) spread Greasiness Non-greasy Very slightly Slightly Greasy Very greasy greasy greasy Washability Very Slightly Very slightly Practically Non-washable washable washable washable non -washable falo, NY) to a thickness of approximately 200 }xm. The sectioned skin was stored in normal saline solution in a refrigerator until ready to use within 24 hr. IN VITRO HUMAN SKIN PERMEATION STUDIES Flat-type 9-mm inside diameter Franz diffusion cells with open top caps were used. Normal saline solution maintained at 37 --- 0. iøC was used as receptor fluid. A piece of section skin was carefully mounted on top of the cell. A known weight of test formula (approx. 50 mg) was applied on the skin surface at the center of the cell covering the entire cell, then the cap was secured in place with a clamp. One hundred and fifty •xL of sample was drawn at the following intervals: 1, 4, 8, 24, 26, 28, 30, 32, 48, 50, 52, 54, 56, and 72 hr. The withdrawn volume was replaced with fresh normal saline to maintain the initial volume. The sample solutions were immediately ready for HPLC analysis. The skin specimen was examined visually to ensure that no leakage occured during the experiment. CALCULATION OF PERMEABILITY CONSTANT When the cream is present on the surface of the specimen, the concentration of drug in the outer layers of the stratum corneum at equilibrium is higher than the concentration in the receptor fluid. The concentration in the lower layers of the skin remains near zero, since these layers are in contact with a fluid that is being continuously replaced or through which diffusion is relatively rapid. The flux, therefore, is more accurately re- lated to the difference in concentration between the top and the bottom layers of the skin (15). The concentration in the top layers of the skin is determined by the relative solubility of the drug in the vehicle and the skin, i.e., the partition coefficient of drug in the skin (Kin). Fick's law is then expanded to:
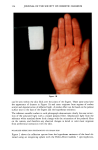
EMULSION VEHICLES AND VASOCONSTRICTOR ACTIVITY 145 dQ AKmDC o - (1) dt h where dQ/dt is the flux h is the thickness of the skin Q, the amount of drug diffused into the receptor fluid at time t D, the diffusion coefficient of the drug in the skin and A, the area of the drug applied on the skin. Equation (1) can be rearranged to, dQ/dt _KmD (2) Kp -- C O A h where Kp is the mean permeability constant. IN VIVO VASOCONSTRICTOR STUDIES The vasoconstructor activity of hydrocortisone 17-valerate 0.2% in the finally selected emulsion was evaluated clinically and compared to that of other marketed corticosteroid products in healthy human volunteers. The method used was similar to the vasocon- striction assay described by Burdick eta[. (16), which is a modification of the bioassay described by McKenzie and Stoughton (17). Approximately 3 mg of each preparation was applied to 12-16 sites (each 7 mm x 7 mm in size) on the volar surface of the forearms of 24 normal healthy subjects. The materials remained on the skin for six hours, unoccluded but protected from mechanical abrasion by an elevated plastic arm guard. A restricted randomization process, balanced for sites and arms, was used in assignment of preparations to the sites in groups of 8 or 12 subjects. One or two pairs of observers independently evaluated the blanching re- sponses, on a scale of 0 to 3, at 7, 9, 11, 13, and 24 hr after application of the materials, in three separate assays. The results of the vasoconstrictor studies were as- sayed by using the area under the time-response curves according to the trapezoidal rule. The data were evaluated by analysis of variance, using Duncan's procedure for multiple comparisons with p • 0.05 for adjacent pairs of means. RESULTS AND DISCUSSION FORMULATION EVALUATION Thirty-six hydrocortisone 17-valerate 0.2% o/w emulsions (see Table I) were studied. The three major ingredients -- petrolatum, mineral oil, and water--which most likely affect the occlusivity of the emulsion system were treated as variables in the formulation study while other ingredients were held constant. Preparation of formulations with low water content (8.9% and 18.9%) was found to be difficult, presumably because the amount of emulsifiers in these formulations was insufficient for the high oil content formulas. The combination of three variables gives different phase-volume ratios for formulations (see Table I). PHYSICAL STABILITY EVALUATION Based upon the scoring system (see Table II), it was observed that formulas with low water content (4) = 3.59) were not physically stable for three weeks at 55øC, ! month
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)