OXYRADICALS FROM PHOTOIRRADIATED HAIR 171 oo + OH- -- r r OO- H COO- Terephthalate (TA) (Non-fluorescent) 2-hydroxyterephthalate (HTA) (Fluorescent: 3.ex = 315 nm, •'em -- 425 nm) Scheme 1 and reaction with TA to demonstrate and assess the involvement of oxygen radicals when hair is irradiated at wavelengths above 320 nm. EXPERIMENTAL MATERIALS All chemicals were reagent grade quality and were used without further purification. Terephthalic acid, sodium hydroxide, and potassium phosphate monobasic (KH2PO4) were purchased from Fisher Scientific Company (Fair Lawn, NJ). DMPO (5,5-dimethyl- 1-pyrroline N-oxide), 2-bromoterephthalate, and sodium azide were purchased from Sigma (St. Louis, MO). Sodium benzoate, and sodium phosphate dibasic (Na2HPO 4 ß 7H:O) were from Allied Chemical (New York). Ethanol (200-proof) was from Quantum Chemical (Tuscola, IL). Standard 2-hydroxyterephthalate (HTA) was synthesized from 2-bromoterephthalate by the method of Mason et al. (27). The crude precipitate was purified using liquid column chromatography until the fluorescence spectrum matched that from the literature (28). The melting point of the purified crystals is 321-325øC (lit. m.p. 320-322øC). This authentic HTA was used for the standard curve. For the HTA calibration curve, a 2.0 mM HTA (pH 7.6) stock solution was diluted with 50 mM phosphate buffer (pH 7.6). The standard curve was obtained by plotting fluo- rescence intensity at 425 nm against HTA concentration (25) it is linear up to at least 10 pM. The milled keratin powder was provided by Zotos Corporation. Hair tresses were pur- chased from DeMeo Brothers. Before each experiment, the hair samples were washed with 4.5 % sodium lauryl sulfate and doubly distilled water, and left at room tempera- ture to dry. The clean and dry hair sample was cut into 1-mm-long pieces. The cut hair or wool powder was added to a 1- or 2-ml buffered solution of ESR spin trap (DMPO) or fluorescence probe (TA) in a 1-cm square Pyrex spectrometer cuvette for irradiation. Samples were normally stirred to maintain air saturation.
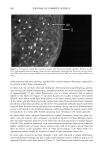
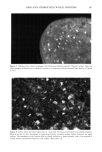
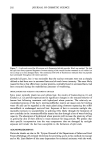
172 JOURNAL OF COSMETIC SCIENCE All irradiation was done with a 150-W xenon lamp through glass filters transmitting UVA and visible light (--320 nm). The measured light flux falling on 50% of a 2-ml sample was typically 0.35 mw/cm 2 (+ 15%). After irradiation, each sample was filtered through a 0.22-}am filter, and the flitrate was transferred to an ESR quartz fiat cell for ESR measurements or to a fluorescence cell for fluorescence measurements. To minimize the effects of varying lamp intensity and other factors, series of experiments comparing different samples were always done on the same day. Replicate experiments performed on different days were generally within + 10% for HTA or +20% for ESR experiments, but relative trends for different types of hair were always the same. INSTRUMENTAL Electron spin resonance (ESR) measurements were performed at room temperature on a Bruker ER200 spectrometer operating at 9.76 GHZ with 100 KHZ field modulation and microwave power of 20 mW. Direct ESR spectra of hair were taken in 3-mm quartz tubes. For the spin trapping experiments, irradiation (in 1-cm cuvettes) was done outside of the ESR cavity, and samples were transferred to the fiat cell after irradiation. While irradiation of melanin within the ESR cavity permitted detection of the superoxide radical (25), the instability of DMPO-O 2- allows only DMPO-OH to be observed in the present experiments. Fluorescence spectra were taken with a Spex Fluorolog-1680 spectrometer in 1-cm disposable plastic cells. HTA signals (kex = 315 nm, )kem= 425 nm) were identified and quantified by comparison with synthetic HTA. All fluorescence measurements reported here are on the same arbitrary scale, with a value of 10 corresponding to 0.1 }aM of HTA. Because of absorption, fluorescence, and/or fluorescence quenching, individual reference solutions were prepared for each experiment containing the same concentrations of all components except hair (or keratin). Irradiated solutions of HA in the absence of hair or other additives did not give significant production of HTA. RESULTS AND DISCUSSION The intrinsic ESR signal for a variety of hair types, as well as for milled wool keratin, is shown in Figure 1. The pigmented black, brown, and red hair exhibits comparatively large signals (g = 2.004), due essentially to the presence of melanin. The broader and much weaker signals from blond, white, and bleached hair have a slightly lower g value and are similar to those of milled keratin. Figure 2 shows the buildup and decay of melanin signals from brown and red hair. The increase of the intrinsic melanin signal under irradiation for the red hair is smaller than that for the brown hair, a difference which we also noted with suspensions of extracted melanins (25). Figure 3 demonstrates that when wool keratin is irradiated with UVA and visible light in the presence of terephthalate ion, HTA is formed, indicating that hydroxyl radicals are produced during this process. The production of HTA is quenched when ethanol or sodium azide is added to the reaction mixture. Both ethanol and sodium azide are hydroxyl radical scavengers, and thus compete with TA for the hydroxyl radicals produced when the keratin is irradiated. Human hair has a structure similar to that of wool (5). When hair is irradiated in the presence of TA, HTA is also produced. Figure 4 compares the yield of HTA from different varieties of hair after irradiation for 90 minutes. As in our previous study (25), we observe a steady increase in HTA with increasing irradiation
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)