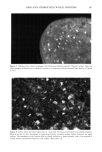
NEAR-INFRARED SPECTROSCOPY 191 (1) .40 .30 ,) .10 (6) .20 1050 1100 1150 1200 1250 1300 1350 Wavelength (nrn) Figure 5. Absorption spectra of human hair. The spectra, from the top, represent: (1) hair from one of the authors (C.P.) (2) commercially blended medium brown hair (3) commercially blended gray hair (4) commercially blended gray hair dyed with product A (5) commercially blended gray hair dyed with product B and (6) Piedmont hair. The spectra represent the tail end of melanin absorption. Comparison of spectra I and 6 reveals the absorption due to melanin pigment, while comparison of spectra 3, 4, and 5 reveals the "lift" produced by permanent hair dyes. Notice that product B provides slightly more "lift" compared to A, even though they are both dark brown shades. Such subtle differences between products translate into differences in the appearance of hair color and wearing properties. spectrum 5 produced more bleaching (lift) than the one responsible for spectrum 4. Thus, this methodology can be used during the product development process to opti- mize lift to the desired level, based on the market positioning of the product. It should be recognized that conventional UV/visible reflectance measurements would be more sensitive than the NIR methodology described above for monitoring chemical or photochemical bleaching, due to higher extinction in this region of the spectrum. Such instrumentation, however, cannot distinguish between natural pigment color and syn- thetic colors. In summary, we have evaluated NIR spectroscopy for use in hair research. We show that it will prove to be a valuable tool in hair care and hair color product research, devel- opment, and claims substantiation. This fiber-optic-based instrumentation is capable of measurements on live heads, which also makes it suitable for salon use. REFERENCES (1) C. Robbins, Chemical and Physical Behavior of Human Hair (Springer-Verlag, New York, Berlin, Heidelberg, 1988).
192 JOURNAL OF COSMETIC SCIENCE (2) I. C. Watt, Sorption of water vapor by keratin,J. Macrotool. Sci. Rev. Macrotool. Chem., C18(2), 169-245 (1980). (3) B. Forslind, in Hair and Hair Disease, C. E. Orfanos and R. Happle, Eds. (Springer-Verlag, New York, Berlin, Heidelberg, 1980), pp. 73-97. (4) J. D. Leeder and I. C. Watt, The role of amino groups in water absorption by keratin. J. Phys. Chem., 69, 3280 (1965). (5) L.J. Pauling, The adsorption of water by proteins, J. Amer. Chem. Soc., 67, 555 (1945). (6) S.J. Smith, The sorption of water vapor by high polymers, J. Amer. Chem. Sot., 69, 646 (1947). (7) P.R. Crippa, V. Cristofoleti, and N. Romeo, A band model for melanin deduced from optical absorption and photoconductivity experiments, Blochim. Biophys. Acta., 538, 164-170 (1978). (8) P. L. Walling and J. M. Dabhey, Moisture in skin by near-infrared reflectance spectroscopy, J. Soc. Cosmet. Chem., 40, 151-171 (1989). (9) K. Martin, Direct measurement of moisture in skin by NIR spectroscopy, J. Soc. Cosmet. Chem., 44, 249-261 (1993). (10) K. Martin, In vivo measurements of water in skin by near-infrared reflectance, Appl. Spectroscopy, 52(7), 1001-1007 (1998). (11) J. deRigal, M. J. Losch, R. Bazin, C. Camus, C. Sturelle, V. Descamps, and J. Leveque, Near-infrared spectroscopy: A new approach to the characterization of dry skin, J. Soc. Cosmet. Chem., 44, 197-209 (1993). (12) Y. Ozaki, T. Miura, K. Sakurai, and T. Matsunaga, Nondestructive analysis of water structure and content in animal tissues by FT-NIR spectroscopy with light-fiber optics. Part I: Human hair, AppL Spectroscopy, 46(5), 875-878 (1992).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)