Cosmet. Sd., 51, 193-203 (May/June 2000) Investigations on the penetration of hydrolyzed wheat proteins into human hair by confocal laser-scanning fluorescence microscopy J. A. SWIFT, Department of Textile Design & Prodution, De Montfort University, The Gateway, Leicester, LE1 9BH, England and S. P. CHAHAL, N. I. CHALLONER, and J. E. PARFREY, Croda Colloids Ltd., Foundry Lane, Ditton, Widnes, Cheshire, WAS SUB, England. Accepted for publication March 15, 2000. Synopsis Hydrolyzed wheat proteins, of a type commonly used in hair toiletry products, were labeled by reaction with fluorescein isothiocyanate. Aqueous solutions were applied to untreated hair and also to hairs treated beforehand by permanent waving, by bleaching, or by relaxers. The extent of penetration of the peptides into the hairs and their structural locations in them were determined by imaging transverse sections of the resin-embedded hairs with a confocal laser-scanning fluorescence microscope. Experimental evidence and arguments are mustered that the fluorescently labeled peptides, having been purified, provide a satisf•tctory model for studying the diffLsional behavior of the native peptides in hair. Penetration of the hydrolyzed wheat proteins into all the hairs was extensive. The main sites for occupation of the peptides were the endocuticle and in the cortex, the nuclear remnants (very intense), intermacrofibrillar matrix, and along the cell boundaries. INTRODUCTION The absorption of partially hydrolyzed wheat proteins (Cropeptide W TM, hydrolyzed wheat protein supplied by Croda Oleochemicals, Snaith, Goole, England, and Croda Inc., Parsippany, NJ) into human hair from aqueous solution was shown by Gamez- Garcia (1) to alter the tensile mechanical properties of the fibers, notably as they were being dried. He found that hairs rapidly stretched by 1% at constant relative humidity, briefly immersed in water, and then withdrawn to an atmosphere at the same relative humidity, took four minutes to recover 80% of their initial tensile stress. On the other hand, for a similar experiment carried out with a solution of the hydrolyzed wheat protein, recovery to 80% initial stress took many hours. That such a dramatic effect might be due to the ability of hydrolyzed wheat proteins to impede the loss of water Address all communications to Dr. J. A. Swift, 29 Moorland Park, Gayton, Wirral, CH60 8QJ, England. 193
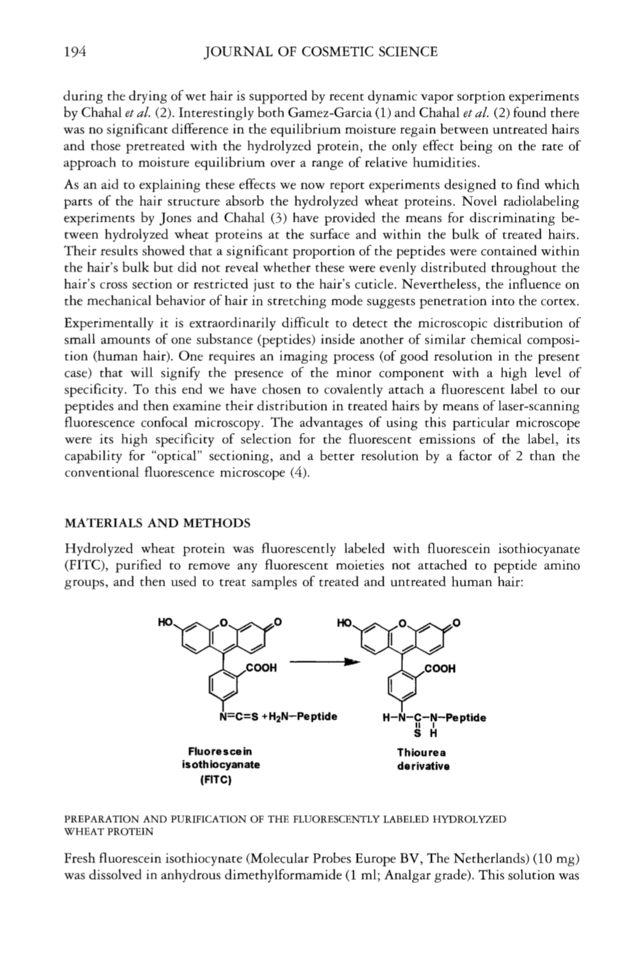
194 JOURNAL OF COSMETIC SCIENCE during the drying of wet hair is supported by recent dynamic vapor sorption experiments by Chahal et al. (2). Interestingly both Gamez-Garcia (1) and Chahal et al. (2) found there was no significant difference in the equilibrium moisture regain between untreated hairs and those pretreated with the hydrolyzed protein, the only effect being on the rate of approach to moisture equilibrium over a range of relative humidities. As an aid to explaining these effects we now report experiments designed to find which parts of the hair structure absorb the hydrolyzed wheat proteins. Novel radiolabeling experiments by Jones and Chahal (3) have provided the means for discriminating be- tween hydrolyzed wheat proteins at the surface and within the bulk of treated hairs. Their results showed that a significant proportion of the peptides were contained within the hair's bulk but did not reveal whether these were evenly distributed throughout the hair's cross section or restricted just to the hair's cuticle. Nevertheless, the influence on the mechanical behavior of hair in stretching mode suggests penetration into the cortex. Experimentally it is extraordinarily difficult to detect the microscopic distribution of small amounts of one substance (peptides) inside another of similar chemical composi- tion (human hair). One requires an imaging process (of good resolution in the present case) that will signify the presence of the minor component with a high level of specificity. To this end we have chosen to covalently attach a fluorescent label to our peptides and then examine their distribution in treated hairs by means of laser-scanning fluorescence confocal microscopy. The advantages of using this particular microscope were its high specificity of selection for the fluorescent emissions of the label, its capability for "optical" sectioning, and a better resolution by a factor of 2 than the conventional fluorescence microscope (4). MATERIALS AND METHODS Hydrolyzed wheat protein was fluorescently labeled with fluorescein isothiocyanate (FITC), purified to remove any fluorescent moieties not attached to peptide amino groups, and then used to treat samples of treated and untreated human hair: N=C=S + H2N-Pe ptide H-N-C-N-Pe ptide II I s H Fluorescein Thiourea is oth iocyanate d e rivative (FITC) PREPARATION AND PURIFICATION OF THE FLUORESCENTLY LABELED HYDROLYZED WHEAT PROTEIN Fresh fluorescein isothiocynate (Molecular Probes Europe BV, The Netherlands) (10 mg) was dissolved in anhydrous dimethylformamide (1 ml Analgar grade). This solution was
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)