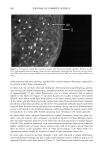
HAIR AND HYDROLYZED WHEAT PROTEINS 197 blotted dry between tissue paper, and dried overnight in a desiccator over silica gel. Fibers from each sample were individually stretched between slits in small silicone molds, acrylic embedding resin "LR White" (London Resin Co., Reading, England) was poured in, and the fibers polymerized at 60øC for 24 hours. Two slightly different methods were used for preparing specimens for the microscope. In one case transverse sections of 25-ylm thickness were cut from each block on a steel knife, using a regular histology microtome, and these were laid onto appropriately labeled individual micro- scope slides. In the other case, the resin block used for the preparation of the sections was trimmed further to define an area of about 300-1•m square around each fiber. This transverse surface was then smoothly faced using a freshly prepared glass knife on a Reichert UM2 ultramicrotome to pare off sections of gradually decreasing thickness down to 50 nm. Each resin block (of ca. 10 mm height) was glued to a microscope slide with the smoothed face uppermost and, as near as could be judged by the eye, parallel with the plane of the slide. All the microscopy work was carried out using a Biorad MRC600 laser-scanning con- focal attachment to a Nikon Optiphot light microscope. This provided the capability not only for confocal laser fluorescence imaging but also for transmission imaging with tungsten or UV light sources and a selection of different microscope objective lenses. The section and block tops were examined initially at low power under tungsten illumination and sometimes using UV. Once a hair had been located, it was centered in the field, a drop of immersion oil was added, and then the hair was imaged in the laser-scanning confocal mode at higher magnifications using a x60 1.4 NA oil-immersion objective. The specimen was scanned with a beam from an argon-ion laser (•kma x = 488 rim, spot size 0.5 l•m), and the system was tuned to provide images exclusively from the fluo- rescent emissions of the fluorescein-labeled peptides (at h. -- 518 nm) within an optical slice of the specimen between 0.5 and 1.0 lnm thick. The vertical position of the specimen was adjusted so that optical slicing was just below the upper surface of the sections and of the smoothed block tops. Image magnification was varied according to changes in the overall dimension of the laser-scanned field. Images were averaged by Kalman filtering (30 frames) to improve their signal-to-noise content and stored on computer disk. RESULTS AND DISCUSSION The various types of hair were treated with the fluorescent probe specifically attached to the target peptides and free from either unreacted FITC or FITC decomposition products as demonstrated by size-exclusion HPLC analysis. Since the peptides contain only 1.46 mole% of lysine, most of the fluorescein level will be covalently attached to the end- amino groups of the peptides. A major concern was that the addition of the fluorescent probe to the hydrolyzed wheat proteins should not affect their diffusion behavior in hair. The molecular mass of each peptide fragment will have increased by 389 Da in its reaction with FITC, accounting for a 38.9% increase for peptides of average mass (ca. 1000 Da) in the initial mixture. The reaction will also have eliminated one amino function and introduced one new carboxyl function into each peptide unit. The starting peptides already contain 40% (by weight) of their amino acid residues as glutamic and aspartic acids (37% and 3%, respectively), or 36 mole%, and so their anionic character will have been enhanced in the
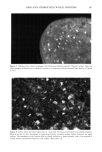
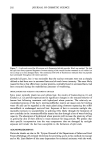
198 JOURNAL OF COSMETIC SCIENCE fluorescein conjugates. Thus for peptides of average molecular mass and containing eight amino acid residues, the effective side-chain carboxyl function will have increased from an average of four carboxyl groups (including the terminal group) per peptide chain to five carboxyl groups per chain. While the original peptides have clearly been altered, the overall changes are not considered to be excessive. We believe therefore that the fluo- rescently labeled material will have provided a realistic model for studying the diffusion of unlabeled wheat protein hydrolysates into hair. Both methods of specimen preparation yielded satisfactory information about the pen- etration of the fluorescently labeled peptides into the various hair types. The confocal images showed the hair's internal structure with a clarity (i.e., resolution) significantly better than is normally provided by the conventional fluorescence microscope. It was possible, for example, to identify the hair's endocuticle by the irregularity of its outer- facing surface and the smoothness of the inner-facing surface. Both methods for pre- senting specimens to the microscope, yielded similar information. The hairs in the physical sections were often torn (Figure 2), whereas the smoothly planed block tops were free from such imperfections (Figure 3) and were thereby a preferred method of hair sample presentation. All samples (untreated ones and those pretreated by various cosmetic processes) con- tained the labeled peptides in discrete structural locations throughout the entire cross section of each hair (Figures 2-7). For the undamaged root-end hairs (Figure 2), lesser amounts of fluorescer were contained within samples treated with the peptides for 30 minutes than in those treated overnight (Figure 3). In both, the highest levels occurred in the cuticle and peripheral cortex, and from there the intensity fell off rapidly inwards. Figure 2. Root-end hair treated with fluorescein-labeled peptides for 30 minutes. Physical section. Fluo- rescence throughout the hair, but higher concentrations at its periphery. Arrows indicate tear in the section (T).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)