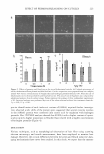
262 1.44 1.42 t1 � 1.4 � � 1.38 ;.. ·-= � 1.36 1.34 1.32 o JOURNAL OF COSMETIC SCIENCE 20 40 ----■� RI Hexylg Ext RI Hexylg Cal 60 80 Percentage of Hexylene Glycol 100 Figure 4. Theoretical and experimental concentration-dependent RI values of hexylene glycol aqueous solution. Solid line is experimental and dashed line is theoretical. ecules, between glycerin molecules, and also between water and glycerin molecules. The interaction between water molecules and between glycerin molecules is possibly stronger than the interaction between water and glycerin molecules. A decrease in specific gravity results in a decrease in optical density, which is observed as a negative deviation in refractive index measurements. It turns out that glycol solutions behave differently compared to glycerin solutions, as is illustrated in Table IV. The observed increase in specific gravity corresponds to a decrease in volume after glycols dissolve in water. A plausible explanation could relate to spatial filling, since the three glycol molecules are structurally larger than water molecules. Also, water molecules can fill voids between glycol molecules in glycol solution. An increase in solution specific gravity leads to an increase in optical density, which results in positive deviation in refractive index measurements. Propylene glycol, butylene glycol, and hexylene glycol all show positive deviation (Fig ure 2). However, propylene glycol demonstrates the least and hexylene glycol the most. The nature of deviation is related to molecular interaction between water molecules, between glycol molecules, and between water and glycol molecules. These three glycols have the same diol (dihydroxy) functional group on two carbon atoms (illustrated in
1.48 1.44 � 1.4 ·-= "' e � � 1.36 1.32 0 REFRACTIVE INDEX MATCHING 20 40 -•- Glyrth-7 Exp Glyrth-7 Cal 60 80 Percentage of glycereth-7 263 100 Figure 5. Theoretical and experimental concentration-dependent RI values of glycereth-7 aqueous solution. Solid line is experimental and dashed line is theoretical. Glycol Glycerin Hexylene glycol Butylene glycol Propylene glycol Table IV Specific Gravity of Glycols in 50% Aqueous Solution SPG Exp SPG Cal 1.1268 1.1304 0.9870 0.9613 1.0251 1.0085 1.0354 1.0184 �SPG -0.0036 +0.0257 +0.0166 +0.0170 Figure 6). The more carbon contained within the molecular structure, the bulkier the glycol molecule. The more carbon the molecule has, the greater the difference will be in molecular interaction between glycol and water molecules. The greater this difference, the more positive is the observed deviation (Figure 2). Hexylene glycol shows the greatest increase in specific gravity and a concomitant positive deviation in the refractive index. Negative RI deviation corresponds to a decrease in the specific gravity of glycerin solution. Positive RI deviation corresponds to an increase in the specific gravity of glycol solution. Based on these observations, it is reasonable to generalize that any
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)