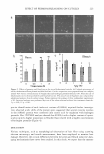
iii )I( Ill D. VITAMIN C AND SDS/PENTANOL/WATER SYSTEM 273 0.03 ----.----........----------.-----,.-----,------...-----, 0.025 I I I I I I - - - - -- -- - - -- - , - -- - - - - I - - - -- - -- , - - - - - - I - --- - - T - -- - - - -- , - - 0.02 --2%vit.C i 0.015 • _. - 10% vit. C . .. . . . .. : ..... : .:i-_. �· � '- . B C i- - - - - -:- - . ·g CJ Cl) iii 0.01 0.005 -• 20%vit.C -X• 60%vit. C I I I "- I I ! I I llii,. I I I � : 0/W -- ____ ..!_ _______ 1 _______ 1 ____ -- -'------ _!..._ _ -- _ -- .!_ ______ -·- _ ---- __ 1_ -- --__ 1 __ -- _ -- ' I I : ... : I I .. : I ' .... ' ' ' ' ' -, I I I 'lill, ' .. ' - - ! - - - o._ ________ .,.. __ ..., _____ ...,. ______ .,... __ .,..._----111 0 10 20 30 40 50 60 70 80 90 100 Water Content in Microemulsion (Wt%) 0.03 ------------.-----.-----,.----,.-----,.----...----...-----, d) 4�°C 0.025 , I I I I l J - - , - ---- - - , - - - - - -- 1 - - - - - - - T -- - -- - - 1 -- - - - - - 1 - - - - - - - 1 - - - - - - - 0.02 f t I I I I - - -- - -- 1 - - - - -- - 1 - - - - - - - .- - - - - - - - . - --- -- - . - - - - - - -- , -- - -- - -+--no vit. C W/0: i 0.015 --2%vit.C ' ' .. • • 10% vit. C · · · · · - ·, - - - - - - -,- - - - - · 'g CJ Ill 0.01 0.005 0 -• 20%vit.C --X - 60% vit. C • 10 20 30 40 50 60 70 Water Content in Microemulsin (Wt %) Figure 4. (cont'd.) 80 90 100 All rheological measurements were conducted with a Bohlin Rheometer CVO 50 with a PC computer and thermostat RTE 111 M (Spectro-Lab). The double-gap measuring system DG 24/27 consists of a hollow cylinder, which is lowered into a cylindrical groove
274 JOURNAL OF COSMETIC SCIENCE in the outer cylinder. The sample of microemulsion is contained in the double annular gap between them. The time of measurement was 195 7 s, shear stress CJ was changed in the range of 3. 24 · 1 0 - 2 - 64 mPa, and shear rate --y was changed from 2 . to 5. 0 s - 1 . RESULT AND DISCUSSION VISCOSITY OF MICROEMULSION FORMED IN THE SDS/PENTANOL/WATER SYSTEM The viscosity of the studied system as it goes from the isotropic phase through a lamellar mesophase regime and again to an isotropic phase at high water content is presented in Figure 2. Microemulsion viscosity increases with increasing water content in the system beginning with the value characteristics for pentanol (4.65 mPa · s for l 5°C 2.99 mPa · s for 30 ° C) (40 ). In an inverse micellar solution, the basis for W/0 microemulsion, it reaches a plateau at the value of water concentration, depending on the temperature (40 wt% of water for 15°C 1 0 wt% of water for 40 ° C), which finishes at 72 wt% of water content ( in a bicontinuous part of the phase diagram). Next, the viscosity of the aqueous micellar solution that forms the basis for the 0/W microemulsion decreases and finally reaches the value close to that characteristic for water (Table II). As expected, the increase in the temperature of the system (15 ° C, 22 ° C, 30 ° C, and 40 ° C) caused the microemulsion's viscosity to decrease. These data are in accordance with the well known theoretical fact that the viscosity of material is usually found to decrease with an increase in temperature, assuming no physical/chemical changes are being induced by the applied heat energy (4 2 ,43). Figure 3 presents three examples of so-called flow curves: Figure 3a for the water-in-oil microemulsion (6% SDS, 65 % pentanol, 29% water), Figure 36 for the bicontinuous system (6% SDS, 22% pentanol, 72% water) and Figure 3c for the oil-in-water micro emulsion (6% SDS, 1 % pentanol, 93 % water). For W/0 and 0/W microemulsions the measured viscosity of fluid remains constant independent of the shear rate. These systems demonstrate Newtonian behavior (Figures 3a and 3c). For the bicontinuous system, viscosity decreases as the shear rate is in creased-shear thinning behavior (Figure 36). O ne can notice that the experimental viscosity values are changing in the same manner as those typical for the emulsion system described by Israelachvili (13). The fact outlined above that the bicontinuous phase exhibits non-Newtonian behavior is connected with the large-scale structure in a material. From the statistical picture of a bicontinuous microemulsion, the structure behaves similarly to a highly crosslinked polymer, and consequently rheology similar to that of swollen gels might be expected. However, the microemulsion structure is rapidly fluctuating so that it flows easily, and although snapshots of a polymer gel and a bicontinuous phase might be topologically similar, their dynamic behavior is not at all so (4 4,45). INFLUENCE OF ASCORBIC ACID (AA) ON THE RHEOLOGICAL PROPERTIES OF THE MICROEMULSION REGION OF THE SDS/PENT ANOL/W ATER SYSTEM As follows from our own experiments, ascorbic acid is dissolved/solubilized up to 6 0%
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)