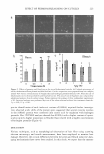
264 JOURNAL OF COSMETIC SCIENCE glycerin propylene glycol butylene glycol hexylene glycol Figure 6. Molecular structures of glycerin, propylene glycol, bucylene glycol, and hexylene glycol. increase or decrease in specific gravity leads to an increase or decrease in optical density, which results in RI values that deviate positively or negatively from calculated values, respectively. The dominant interaction forces between molecules of water and glycols or glycerin are hydrogen bonds since all of them are polar molecules with hydroxyl groups. Hydrogen bonds play important roles for the decrease in specific gravity in glycerin solution. The reason for volume increase (specific gravity decrease) when forming glycerin aqueous solution is that there is stronger hydrogen bonding between glycerin molecules than the hydrogen bonding between molecules of water and glycerin. That the process accom panies a significant heat release when hydration takes place also indicates the different strength of hydrogen bonding. Glycols, however, are hydrated differently. That there is a volume decrease (specific gravity increase) when forming glycol solutions indicates that there is weaker hydrogen bonding between glycol molecules than the hydrogen bonding between molecules of water and glycols. It is apparent that the most efficient way to raise the refractive index of the aqueous phase is to use a combination of hexylene glycol in the range of 20-30% with either glycerin, glycereth-7, or butylene glycol. Propylene glycol is less efficient at raising the RI of the water phase because it has the least positive deviation and the lowest refractive
REFRACTIVE INDEX MATCHING 265 index value in the group. Glycereth-7 is also the easiest one to use in calculation since it has the least deviation from calculation. In the formulation process one has to consider, among other factors, the formulation's performance, ingredient cost, and ease of opera tion. CONCLUSIONS By using Snell's law in cosmetic formulation, a simple calculation scheme has been developed for designing clear emulsion formulas by matching the refractive indices of the water phase and the oil phase. The RI value of the water phase was adjusted by varying the ratio of water and glycols. Positive deviation and negative deviation were observed for water-glycol two-component systems. The optical density changes in glycol aqueous solutions result in RI deviation. The most effective glycols are the glycereth-7 and glycereth-26 type, which have the least deviation from calculation. The use of index calculation and deviation charts leads to more precise formulation design. REFERENCES (1) L. M. Prince, Ed., Microemulsions, Theory and Practice (Academic Press, New York, 1977), pp. 3-55. (2) Using Silicone Formulation Aids to Formulate Cosmetic System (Dow Corning, 1995), pp. 6-7. (3) N. M. Karassik, P. P. Angelone, Jr., P. R. Boyle, P. Domizio, C. W. Galante, J.C. Patel, and P.A. Patricia, US Patent 5,925J338, the Gillette Company, 1999. (4) J. Z. Sun, M. C. E. Erickson, and J. W. Parr, Refractive index matching: Principle and cosmetic applications, Cosmet. Toiletr., 118, 65-74 (2003). (5) E. Hecht, Physics (Brooks/Cole Publishing Company, 1996), pp. 935-960. (6) A. Marcin, P. Bustamante, and A. H. C. Chun, Physical Pharmacy (Lea & Febiger, Philadelphia, 1993), pp. 95-96. (7) K. Kostarelos, T. Tselepi, and A. Teknetzis, AHA and exfoliative skin diseases, Connet. Toiletr. 114, 43-52 (1999). (8) D.S. Orth, J. Widjaja, L. Ly, N. Cao, and W. B. Shapiro, Stability and skin persistence of topical products, Cosmet. Toiletr. 113, 51-64 (1998). (9) S. M. Starch, Screening silicones for hair luster, Cosmet. Toiletr., 114, 55-62 (1999). (10) M. Krenceski, R. Perry, S. Nye, M. Navarrate, and D. Riccio, Role of functionalized silicones in hair shine, SCC Annual Scientific Seminar (Washington, DC, 2003), p. 51.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)