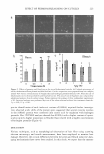
VITAMIN C AND SDS/PENTANOL/WATER SYSTEM 275 in the microemulsion region of the SDS/pentanol/water system (18). The % content of ascorbic acid in the sample ought to be understood as a percent of the total microemul sion weight in this sample. The addition of AA to the system caused reduction of the microemulsion region in the phase diagram. Ascorbic acid serves as a competitive organic anion to the surfactant, changing the W /0 microemulsion system towards an 0/W one. Figure 4 illustrates the influence of ascorbic acid on the rheological properties of the surfactant system. The ascorbic acid addition to the system (2%, 10%, 20%, 60% of the microemulsion weight) caused the increase in the microemulsion viscosity, the highest in the bicon tinuous region (see Figure 4a for 15°C, Figure 46 for 22°C, Figure 4c for 30°C, and Figure 4d for 40°C). One can notice the decrease in the W/0 microemulsion region with the increasing AA concentration, which is consistent with the phase diagram described earlier. As we have already mentioned, the phenomenon of viscosity increasing occurs because the AA presence in the system changes its microstructure. The ascorbic acid, being an electrolyte, causes formation of bigger surfactant aggregates, which contain some amount of the continuous phase. As a result, the volume of the dispersed phase increases, resulting in an increase in the total viscosity of the colloidal system (42). The temperature dependence of viscosity is more clearly seen in Figure 5, where an example for the ascobic acid concentration equal to 20% is presented. There is a great consistency in the results presented above with those discussed earlier (Figure 2) and with the typical viscosity/temperature curves for microemulsions known from data in the literature (13,42,45). 0.014 -----------------------------------, AA70% 0.012 0 +---➔------1.....--.....--....--..... ---+---....--.....---+----I 0 10 20 30 40 50 60 70 80 90 100 Water Content in Microemulsion (Wt%) Figure 5. Influence of temperature on the viscosity of the microemulsion region of the SDS/pentanol/water system (20% ascorbic acid).
276 JOURNAL OF COSMETIC SCIENCE CONCLUSION From the investigations carried out it can be stated that: 1. The W/O and O/W microemulsion region of the SDS/pentanol/water system behaves as a typical Newtonian fluid: viscosity remains constant for all the shear rates. 2. The ascorbic acid addition to the system causes the viscosity of the microemulsion to increase. The largest Tl increases were observed in the bicontinuous region and at the lowest investigated temperature. The ascorbic acid presence in the system changes its microstructure, which causes changes in the colloidal system's viscosity. REFERENCES (1) T. Beitz, J. Kotz, G. Wolf, E. Kleinpeter, and S. E. Friberg, Poly(N-vinyl-2-pyrrolidone) and 1-octyl- 2-pyrrolidinone modified ionic microemulsions,j. Colloid Interface Sci., 240, 581-589 (2001). (2) P. C. Hiemenz and R. Rajagopalan, "Principles of Colloid and Surface Chemistry, Colloidal Struc tures," in Surfactant Solutions: Association Colloids, 3rd ed. (Marcel Dekker, New York, Basel, Hong Kong, 1997), p. 355. (3) R. Oda, J. Narayanan, P.A. Hassan, C. Monohar, R. A. Salkar, F. Kern, and S. J. Candau, Effect of lipophilicity of the counterion on the viscoelasticity of micellar solutions of cationic surfactants, Langmuir, 14, 4364-4372 (1998). (4) F. Kern, R. Zana, and S. J. Candau, Rheological properties of semidilute and concentrated aqueous solutions of cetyltrimethylammonium chloride in the presence of sodium salicylate and sodium chlo ride, Langmuir, 7, 1344-1351 (1991). (5) R. Makhoufi and R. Cressey, Temperature dependence of the non-Newtonian viscosity of elongated micellar solutions, Colloid Polym. Sci., 270, 1035-1041 (1992). (6) F. Kern, P. Lemarechal, S. J. Candau, and M. E. Cates, Rheological properties of semidilute and concentrated aqueous solutions of cetylttimethylammonium bromide in the presence of potassium bromide, Langmuir, 8, 43 7--440 (1992). (7) J. F. A. Soltero and J.E.Puig, Rheology of the cetyltrimethylammonium tosilate-water system: Linear viscoelastic regime, Langmuir, 12, 2654-2662 (1996). (8) W. J. Kim, S. M. Yang, and M. Kim, Additive effects on the microstructure evolution in hexadec yltrimethylammonium bromide solution and its rheological properties,]. Colloid Interface Sci., 194, 108-119 (1997). (9) P.A. Hassan, S. J. Candau, F. Kern, and C. Monahar, Rheology of wormlike micelles with varying hydrophobicity of the counterion, Langmuir, 14, 6025-6029, (1998). (10) W. J. Kim and S. M. Yang, Effect of sodium salicylate on the microstructure of an aqueous micellar solution and its rheological responses,]. Colloid Interface Sci., 232, 225-234 (2000). (11) S. J. Candau and R. Oda, Linear viscoelasticity of salt-free wormlike micellar solutions, Colloids Surf A Physicochem. Eng. Aspects, 183, 5-14 (2001). (12) A. Knaebel, R. Skouri, J.P. Munch, and S. J. Candau, Structural and rheological properties of hy drophobically modified alkali-soluble emulsion solutions, ]. Polym. Sci. B Polym. Phys., 40, 1985- 1994 (2002). (13) Israelachvili, The science and applications of emulsions-An overview, Colloids Surf A Physicochem. Eng. Aspects, 91, 1-8 (1994). (14) F. Lequeux, Emulsion rheology, Curr. Opin. Colloid Interface Sci., 3, 408--411 (1998). (15) T. G. Mason, New fundamental concepts in emulsion rheology, Curr. Opin. Colloid Interface Sci., 4, 231-238 (1999). (16) S. Peker, K. Bora, and Y. Over, Effect of interfacial properties on drop size distribution of high internal phase ratio emulsions, Colloids Surf A Physicochem. Eng. Aspects, 182, 43-56 (2001). (17) H. A. Barnes, Rheology of emulsions, Colloids Surf A Physicochem. Eng. Aspects, 91, 89-95 (1994). (18) M. Szymula, J. Szczypa, and S. E. Friberg, A comparison between atmospheric and electrochemical oxidation of vitamin C in surfactant system,]. Dispers. Sci. Technol., 23, 1-9 (2002). (19) M. Szymula, Atmospheric oxidation of vitamin C and E in surfactant system,]. Dispers, Sci. Technol., 21, 983-998 (2000).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)