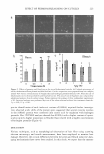
J. Cosrnet. Sci., 56, 219-226 Quly/August 2005) Dissimilar effect of perming and bleaching treatments on cuticles: Advanced hair damage model based on elution and oxidation of S1 OOA3 protein KENJI KIZAWA, TAKAFUMI INOUE, MASAHITO YAMAGUCHI, PETER KLEINERT, HEINZ TROXLER, CLAUS W. HEIZMANN, and YOSHIMICHI IWAMOTO, Basic Research Laboratory (K.K., T.l.) and Cosmetics Laboratory (M. Y.), Kanebo Cosmetics Inc., 5-3-28 Kotobuki-cho, Odawara 250-0002, Japan Department of Pediatrics, Division of Clinical Chemistry and Biochemistry, University of Zurich, S teinwiesstrasse 7 5, C H-80 3 2 Zurich, Switzerland ( P. K., H. T., C. W.H.) and Beauty Care Laboratory, Kanebo Ltd., 134 Kanbe-cho, Hodogaya, Yokohama 240-0005, Japan (Y.l.). Accepted for publication April 4, 2005. Presented in part at the 23rd Congress of the International Federation of the Societies of Cosmetic Chemists, Orlando, Florida, October 24-27, 2004. Synopsis Hair treatment chemicals induce sudden and severe hair damage. In this study, we examined cuticles from untreated, permed, and bleached hair that were mechanically discriminated by shaking in water. Both perming and bleaching treatments are prone to easily delaminate cuticles. Confocal microscopy revealed that the cuticles of permed hair were delaminated with larger pieces than untreated ones. On the other hand, the cuticles of bleached hair tend to fragment into small peptides. At the minimum concentration of thiogly colate required to elute S100A3 protein from the endocuticle into the reductive permanent waving lotion, enlarged delaminated cuticle fragments were observed. Although S100A3 is retained in bleached hair, S100A3 is irreversibly oxidized upon bleaching treatment. It is likely that the oxidative cleavage of disulfide bonds between cuticle-constituting proteins, including S100A3, results in the fragile property of cuticles. Here we present a more comprehensive model of hair damage based on a diverse mechanism of cuticle delamination. INTRODUCTION Although hair damage occurs gradually due to influences of weather and oxidants (i.e., environmental factors) and daily hair care (i.e., physical factors), topical application of Address all correspondence to K. Kizawa. 219
220 JOURNAL OF COSMETIC SCIENCE hairdressing products (i.e., chemical factors) results in sudden and severe changes. Per manent waving and bleaching treatments during hair coloring processes are major causes of hair damage. In order to improve hair care products, it is essential to understand the mechanism of hair damage induced by these procedures. The scale-like cuticle surrounds the hair cortex. Normally, cuticles are damaged prior to changes occurring within the cortex. We previously identified and characterized SlO0A3, a unique member with high cysteine content of the largest calcium-binding SlO0 protein family, characterized by tissue-specific distribution (1), in cuticles (2,3). Its soluble nature under reducing conditions is different from that of other hair proteinous components such as hair keratins and keratin-associating proteins. Ultrastructural lo calization of SlO0A3 in the inner part of cuticles (i.e., endocuticles) suggested its structural role in preserving the attachment of adjacent cuticles (4). The release of S 1 00A3 protein from the attachment site is suggested to be the most relevant event leading to hair damage. We previously proposed a hair damage model that implies the involvement of Sl00A3 in hair damaging processes (5). This model was based on the following four stages: (a) Newly emerging hair is characterized by a smooth-edged cuticle. (6) As a result of cracking of the edge of intact cuticles by normal grooming, the SlO0A3-rich layer in turn becomes the outermost layer. (c) SlO0A3, a soluble protein under non-reducing condition, is oxidized due to the cleavage of inter molecular disulfide bridges under environmental stresses such as UV radiation. (d) Sl00A3 is gradually released from hair during daily washing. It seems also possible that application of permanent waving lotion is able to elute SlO0A3 from natural hair within the second stage. However, it is still unknown how bleaching treatment induces hair damage without elution of SlO0A3. In this study, we examined how chemical hair treatments affected cuticle delamination. Confocal microscopy revealed that permanent waving and bleaching treatments had dissimilar effects on the size of delaminated cuticles. In addition, 2-dimensional elec trophoresis revealed an acidic shift of S 1 00A3 due to irreversible modification of several residues out of the total of ten cysteines in oxidized hair. Based on these results, we present a comprehensive model of hair damage induced by chemical treatments. MATERIALS AND METHODS PERMANENT WAVING AND BLEACHING TREATMENTS Hair was bleached by immersion in 1-10% hydrogen peroxide-ammonia solution (pH 9. 5) for 1 hr at 3 7 ° C. Permanent waving treatment was done by immersing hair at 37 ° C in 0.1-3% ammonium thioglycolate solution containing 1% 2-aminoethanol and 0.45% ammonia for 1 hr, followed by neutralization by 7% sodium bromate for 15 min. Hair samples were collected from males of Japanese descent. DELAMINATION OF CUTICLES The mechanical isolation method for cuticles consisting of stirring hair in water ( 6) was modified with a small-scale delamination test as follows. One-cm hair fibers (200 mg) were stirred at 180 rpm using an R30 shaker (Taitec, Tokyo, Japan) with 10 ml of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)