JOURNAL OF COSMETIC SCIENCE 16 is shown, in turn, to improve skin barrier function, not only in normal but also in dry or irritated skin (2,4,5). Dry skin occurs frequently as a natural disposition in individuals and upon aging, but is also oftentimes due to irritation caused by environmental factors or disease condi- tions such as atopic dermatitis (6). Therefore, skin care products that enhance water retention and skin moisture become important not only for cosmetic purposes but also for treating conditions such as atopic dermatitis. Depending on the type, skin care products function not only by mechanisms in which they form an inert layer on the skin, thus preventing water loss, but also in some cases by penetration and their effect on the skin structure (7). However, the methods to study their functions are limited (8,9). Hydrogenated polyisobutene is a non-drying oil with waterproofi ng properties used in moisturizers (10). Its physical structure allows water retention, making it a poten- tial candidate for a moisturizing emollient. It is a branched-chain aliphatic hydrocar- bon (Figure 1) with an average molecular weight of 370, stable over wide pH ranges and even at temperatures as high as 260°C. Despite its use as a constituent in varied proportions in commercial formulations such as moisturizers (10) and lip gloss treatments (11), information about the actual effects of HP by itself on skin is not fully understood and this is the fi rst attempt to study its isolated functions on skin hydration. For com- parison we tested another commonly used emollient ester, capric/caprylic triglyceride (CCT). CCT is used in food, cosmetic and drug delivery applications, is non-toxic, and exhibits virtually no dermal or ocular irritation (12). Use of CCT in solid nanoparti- cles was shown to enhance entrapment effi ciency of the drug Nimodipine, and varying its proportion aided in controlled release of the drug (13). Thus, in the present study, we attempt to hypothesize the mechanism of HP as a moisturizer in comparison to the emollient ester CCT, with emphasis on its effects on TEWL, skin conductivity, and skin texture. MATERIALS Carbomer (Noveon Inc., Cleveland OH) triethanolamine (Ruger Chemical Co., Linden, NJ) xanthan gum (CP Kelco, Atlanta, GA) and HP, CCT, phenoxyethanol, methylparaben, butylparaben, propylparaben, isobutylparaben, and glyceryl stearate (Lipo Chemicals Inc., Paterson, NJ) were used in this study. Figure 1. Structure of hydrogenated polyisobutene.
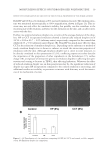
MOISTURIZING EFFECTS OF HYDROGENATED POLYISOBUTENE 17 METHODOLOGY PREPARATION OF SEMI-SOLID FORMULATIONS Three similar simple o/w emulsions were compared in this study. The test formulation and control contained 8% HP and 8% CCT, while the control contained neither of the emollients. Each emulsion was prepared by simple emulsifi cation after heating the aque- ous phase and oil phase to 75°–80°C (Table I). MICROSCOPIC OBSERVATION OF EMULSIONS Emulsions containing HP and CCT were visualized at 400´ magnifi cation using an Olympus BX60 transmission microscope (Olympus Micro Imaging Inc., PA). Emulsion droplet sizes were compared by measuring the mean diameter of the droplets and are presented in terms of relative arbitrary units. STUDY DESIGN Ten healthy female panelists between the ages of 35 and 54 were enrolled, of which there were eight Caucasians, one Hispanic, and one Asian. Panelists were instructed to wash the test sites with Ivory® soap twice daily for three days prior to the study date. On the day of the study, panelists were required to equilibrate in a closed environment with a constant temperature of 70°F and 30% relative humidity for the duration of the study. Biophysical measurements were taken using a Corneometer (Courage-Khazaka, Cologne, Germany) and a Tewameter (Courage-Khazaka), and visual photography was performed by a Charm View video microscope (Moritex USA Inc., Natick, MA). Four test site areas of 50 cm2 on the right and left volar forearms were marked (two on each arm) by using a surgi- cal pen to designate the test sites. One of the test sites was untreated skin. An amount of Table I Composition of Tested Formulations Ingredient HP (% w/w) CCT (% w/w) Control (% w/w) Water 85.12 85.12 93.12 Carbomer 0.11 0.11 0.11 Triethanolamine 0.17 0.17 0.17 Xanthan gum 0.10 0.10 0.10 Phenoxyethanol (and) methylparaben (and) butylparaben (and) propylparaben (and) isobutylparaben 1.00 1.00 1.00 Glyceryl stearate 2.75 2.75 2.75 Cetyl alcohol 1.25 1.25 1.25 PEG-40 stearate 1.00 1.00 1.00 Cetearyl alcohol (and) polysorbate 60 0.50 0.50 0.50 Hydrogenated polyisobutene 8.00 — — Capric/caprylic triglyceride — 8.00 — pH 6.56 6.54 6.60 Viscosity in cps. (LV4@12RPM) 20,000 21,000 15,000
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)