JOURNAL OF COSMETIC SCIENCE 22 Intracellular ROS levels were measured using H2DCFDA, a fl uorescent probe for H2O2. Cells were treated with 20 μM H2DCFDA in HBSS for 30 min. After lysing the cells with 0.1% Triton X-100 in PBS, the fl uorescence (Ex 485 nm, Em 530 nm) was measured using a fl uorescence microplate reader (Spectra Max Gemini Molecular Devices, San Jose, CA). Intracellular ROS levels are calculated as FI per μg protein and are expressed as a fold change of the value of control cells. Protein concentrations were determined using a BCA Protein Assay Kit (Thermo Fisher). Intracellular levels of CPs were determined by fl uorescence labeling of the aldehyde group with FTSC. After fi xation with cold MeOH for 10 min, cells were incubated with FTSC in 0.1 M MES-Na buffer (pH 5.5) and 2 μM Hoechst 33342 for 1 h. CPs and nuclei were quantifi ed by image analysis using corneocytometry software (Ciel, Tokyo, Japan) after obtaining fl uorescence images with a fl uorescence microscope (Floid Cell Imaging Station Thermo Fisher Scientifi c Inc). BARRIER FUNCTION OF POLYSACCHARIDES AGAINST TOBACCO SMOKE Ex vivo study. The barrier function of polysaccharides was characterized by measuring CP levels in corneocytes following exposure to tobacco smoke. Corneocytes were obtained from the upper inner arm of fi ve human volunteers who were nonsmokers by the tape-stripping method using cellophane tape (Nichiban Co., Ltd., Tokyo, Japan) and were transferred to glass slides after dividing each piece of tape into four pieces. One piece of the corneocytes from each volunteer was used for the evaluation of one sample. The corneocytes were treated with 10 μL polysaccharide aqueous solution for 10 min at room temperature. As the control, H2O, which was the solvent for the polysaccharide aqueous solution, was ap- plied on corneocytes. After rinsing with running water and then drying, the glass slides with corneocytes were placed in a box fi lled with tobacco smoke for 2 h at room tem- perature. After a further incubation for 24 h at 37°C, CP levels of corneocytes were mea- sured by fl uorescence labeling as follows: Corneocytes on glass slides were immersed in 0.1 M MES-Na solution (pH 5.5) containing 20 μM FTSC for 1 h at 25°C in the dark. After rinsing, images were obtained using a fl uorescence microscope (Floid Cell Imaging Station, Thermo Fisher). CP levels were quantifi ed from the fl uorescence images using corneocytometry software (Ciel). Informed consent was obtained from each volunteer af- ter explaining the method of collecting corneocytes and the aim of the test. The ex vivo study was approved by the Ethical Committee of the Daito Kasei Kogyo Co., Ltd. and was performed three separate times using the same fi ve volunteers. Statistical analysis. All study data are expressed as means ± standard deviation (SD). Sig- nifi cant differences between experimental values were determined using the Wilcoxon rank-sum test, and p-values less than 0.05 are considered statistically signifi cant. RESULTS PENETRATION OF POLYSACCHARIDES INTO RHEEs In the histological study, it was observed that sacran as well as HA applied topically on RHEEs remained in the stratum corneum (Figure 2). In addition, the amount of sacran
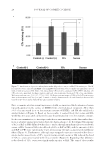
SACRAN PROTECTS SKIN AGAINST POLLUTANTS 23 in the medium of RHEEs topically treated with sacran was approximately 1%, and more than 99% of the sacran was recovered from the RHEE homogenates (Figure 2). HA also showed similar results. These results indicate that polysaccharides topically applied on RHEEs stay in the stratum corneum and do not penetrate well to the living cell layers of the epidermis. TRAPPING EFFECT OF POLYSACCHARIDES ON ACs AND BaP To examine the trapping effects of polysaccharides, the amounts of ACs and BaP in PBS(-) diffused with tobacco smoke passed through membrane fi lters treated with or without polysaccharides were quantifi ed. Sacran-treated membrane fi lters showed excellent trap- ping effects for both ACs and BaP (Figure 3). More than 90% of ACs and BaP were trapped in the sacran-treated membrane fi lter compared with the control (nontreated membrane fi lter). In the case of the HA-treated membrane fi lter, although it failed to signifi cantly trap ACs, it showed a signifi cantly high trapping effect for BaP compared with the con- trol (Figure 3). Thus, although both sacran and HA have trapping effects on ACs and BaP, sacran showed a superior effect compared with HA. SUPPRESSIVE EFFECTS OF POLYSACCHARIDES ON mRNA EXPRESSION OF CYP1A1 INDUCED BY TOBACCO SMOKE HaCaT keratinocytes cultured in DMEM containing PBS diffused with tobacco smoke through a nontreated membrane fi lter showed a predominant upregulation of CYP1A1 mRNA levels. Cells cultured in DMEM containing PBS diffused with tobacco smoke Figure 2. Penetration of biotin-conjugated polysaccharides into RHEEs. RHEEs were treated topically with a biotin-conjugated polysaccharide aqueous solution (sacran or HA) as noted and then were cultured for 24 h at 37°C. The culture media were collected to quantity biotin-conjugated polysaccharides that penetrated through the RHEEs. The histology of biotin-conjugated polysaccharides is shown as representative images (scale bars, 100 μm). The penetration or retention of biotin-conjugated polysaccharides in RHEEs is expressed as a percentage of the amount of biotin-conjugated polysaccharides applied. PBS(-) was used as a control. Each value represents the mean ± SD of three experiments.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)