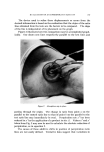
COMPLEX FORMATION IN AEROSOLS 8O7 and an investigation of systems with these surfactants was particularly desirable from a practical viewpoint. Emulsions containing the triethanolamine soaps were prepared as follows: The fatty acid was heated until it melted (about 60-70 øC). An equimolar quantity of triethanolamine was dissolved in the water, and the aqueous solution was heated to about the same temperature as that of the melted fatty acid. The hot aqueous solution was then added slowly with stirring to the fatty acid. After the addition was complete, the mixture was removed from the heat and allowed to cool to room temperature with stirring. When alcohols were included in the formulation, they were melted together with the fatty acid. Aqueous solutions of sodium lauryl sulfate were prepared by dis- solving the surfactant in water. When alcohols were included, they were melted, and the aqueous solution of sodium lauryl sulfate, heated to the same temperature as that of the alcohols, was added with stirring to the alcohols. Concentrations of the Surfactants The concentrations of the surfactants in the aqueous phase were chosen so that the stability of the emulsions and foams from the sur- factants alone would be low. Thus, any effect of the alcohols upon the system would be noticeable. Sodium lauryl sulfate, triethanolamine laurate, and triethanolamine myristate were normally used at concen- trations of 0.10 m in the aqueous phase. On a weight percent basis, this amounted to about a 3.5% solution for these compounds. The addition of sufficient alcohol to produce a 1:1 molar ratio with the sur- factant increased the weight percent to about 4.5%, depending upon the molecular weight of the alcohol. When other concentrations are used, this is noted in the tables. Triethanolamine palmirate and stearate normally were used at con- centrations of 0.025 m in the aqueous phase. This gave solutions of slightly over 1% on a weight basis. The addition of sufficient alcohol to give a 1:1 molar ratio with the alcohol increased the weight percent of the surfactant-alcohol complex to slightly over 2%. Composition of the Aerosol Emulsions Unless otherwise indicated, all aerosol emulsions were prepared with a composition of 90% aqueous phase and 10% Freon-12/Freon-114* (40/60) propellant. In some cases other propellants were tested, and this is indicated in the tables. * Freon is a registered trademark of E. I. du Pont de Nemours and Co., Wilmington, Del.
8O8 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Materials The materials used in the present study were obtained from the following sources: Fatty Acids Laurie acid, myristic acid, palmitic acid and stearic acid were ob- tained from the E. F. Drew Chemical Corp. These acids are marketed under the trade names of "Wecoline" 1295, "Wecoline" 1495, "Wecoline" 1695, and "Wecoline" 1892, respectively. Alcohols Lauryl alcohol ("Lorol" 11), cetyl alcohol ("Lorol" 24), and stearyl alcohol ("Lorol" 28) were obtained from the E. I. du Pont de Nemours and Co. Oleyl alcohol was supplied by Croda, Inc., myristyl alcohol by the Eastman Chemical Corp., and cholesterol by American Choles- terol Products. Sodium Lauryl Sulfate Sodium lauryl sulfate (Duponol* C) was obtained from the E. I. du Pont de Nemours and Co. EXPERIMENTAL RESULTS Sodium Lauryl Sulfate Systems Variation in Alcohols The addition of lauryl, myristyl, or cetyl alcohols to sodium lauryl sulfate systems increased emulsion viscosity and stability and decreased foam drainage. This is shown in Table I. Lauryl and myristyl alco- * Duponol is a registered trademark of E. I. du Pont de Nemours & Co., Wilmington, Del. Table I Variation in Alcohols-Sodium Lauryl Sulfate Syst½•ns • Foam Propcrties Emulsion Properties Drainage Stiffness Density Type of Alcohol b Viscosity Stability (60 min) (g) (g/cc) Discharge None Low 1 min 82 11 0. 055 Quiet Lauryl Medium 5 hr 0 40 0. 064 Slightly noisy Myristyl Medium 5 hr 0 38 0. 068 Noisy Cetyl Low to medium 5 hr 2 14 0. 062 Quiet Stearyl Low 5 min 71 12 0. 071 Quiet Oleyl High 30-60 min 84 10 0. 067 Quiet Cholesterol Low to medium 1-5 •nin 84 13 0. 061 Quiet Sodium lauryl sulfate concentration = 0.10 M. Sodium lauryl sulfate/aleohol ratio (molar) = 1' l.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)