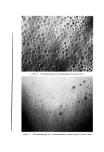
COMPLEX FORMATION IN AEROSOLS 827 increased the bubble size of the foams. It is possible that these effects, which are opposite those of the long-chain alcohols, are due to the for- mation of liquid complexes with cholesterol. Previous work showed that cholesterol formed liquid complexes with sodium cetyl sulfate, while cetyl alcohol formed solid complexes (3). The addition of cholesterol to the triethanolamine-fatty acid systems may have resulted in the formation of a film which had a lower viscosity than that of the triethanolamine salt-fatty acid complex. This would produce a foam with lower stiffness. The increased bubble size would also account for the slight increase in the drainage. Miles and his co-workers showed that the flow of liquids through foams decreased with a decrease in bubble size in the foam (4). The increased bubble size resulting from the presence of cholesterol might be accounted for by the increased fluidity of the liquid films. Such films would be expected to expand more during discharge of the product and subsequent vaporization of the propellant than more solid films. The greater expansion should produce large bubble sizes. Another interesting effect that occurred with the addition of many alcohols was the change from a quiet discharge to a noisy, spurtcry discharge. There probably are a number of factors involved in this effect. If combinations of alcohols and surfactants form strong, solid complexes at the propellant/water interface in the emulsion, these com- plexes might resist expansion when the product was discharged and the propellant vaporized into a gas. The fact that triethanolamine palmi- rate or stearate emulsions also gave noisy discharges without any alcohols present is an indication that fairly strong complexes between the free fatty acid and the triethanolamine soap are formed in these systems. Another factor may be the droplet size of the dispersed propellant in the aerosol emulsion or possibly the uniformity of the droplet size. Emulsions containing large-size droplets might produce a noisier dis- charge than those with smaller droplets. There is no direct evidence for this, but a larger droplet size might explain why some of the cholesterol emulsions gave noisy discharges and also why some propellants gave a product with a noisier discharge than other propellants. SUMMARY AND CONCLUSIONS The effect of various alcohols upon the properties of aerosol emulsions and foams was studied to determine if the alcohols formed molecular complexes with the surfactants. In many cases, the addition of an
828 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS alcohol had a marked effect upon the aerosol system. Emulsion vis- cosity and stability were increased, foam drainage was decreased, and foam stability was increased. Foam stiffness was increased in some systems. On the basis of these results, it was concluded that molecular complexes were formed in aerosol systems. The effect of these com- plexes upon the properties of the systems was similar in many respects to that previously reported with nonaerosol systems. The use of molecular complexes is an effective method for varying the properties of aerosol foams. By the proper choice of surfactant, alcohol, and propellant, foams may be obtained which wet immediately after discharge and then collapse or which wet immediately but retain their foam structure. Foams may also be obtained which are quite stable and show no wetting or collapse for extended periods. Aerosol emulsions can be formulated to give an immediate foam discharge or a liquid discharge which subsequently expands into a foam. The aerosol emulsions were prepared with sodium lauryl sulfate or the triethanolamine salts of lauric, myristic, palmitic, and stearic acid as the surfactants and fluorinated hydrocarbon propellants as the dis- persed phase. The effect of alcohols upon the emulsions and foams was studied with lauryl, myristyl, cetyl, stearyl and oleyl alcohols and cholesterol. The extent to which any alcohol affected the properties of a specific aerosol emulsion or foam depended upon such factors as the type and concentration of the alcohol, the surfactant, and the propellant. The saturated fatty alcohols formed complexes in both sodium lauryl sulfate and triethanolamine-fatty acid systems. Microscopic observation showed that complex formation usually reduced the bubble size of the foams. In some instances, the addition of an alcohol resulted in a product which had a noisy or sputtery discharge. This was attributed to the formation of a solid molecular complex which resisted expansion when the liquefied propellant vaporized during discharge. Cholesterol had little effect in sodium lauryl sulfate systems but formed fluid complexes in the triethanolamine-fatty acid systems. These complexes expanded easily during discharge, and this increased bubble size and decreased foam stiffness. Oleyl alcohol likewise had little effect in sodium lauryl sulfate systems but appeared to form weak complexes in some of the triethanolamine-fatty acid emulsions. The type of propellant had a considerable influence on the properties of surfactant/alcohol systems. In general, the most stable emulsions and foams were obtained with Freon-12, Freon-12/Freon-114 (40/60)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)