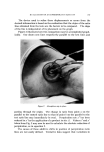
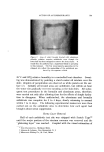
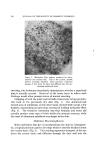
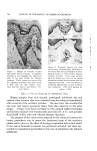
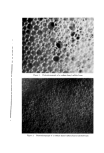
COMPLEX FORMATION IN AEROSOLS Table 11 Variation in Conccntratiou of Lauryl Alcohol in Sodium Lauryl Sulfate Systems 811 Sulfatc"/ Foam Properties •' Alcohol Emulsion Properties Ratio Drainage Stiffness Density (molar) Viscosity Stability (60 rain) (g) (g/cc) 1: 0 Low 1 rain 93 12 0. 056 1: •/• Low 1 min 33 34 0. 059 1: • Low 15-20 min 24 32 O. 062 1: a/• Low 30-60 rain 4 38 O. 063 1:1 Medium 16 hr 0 34 0. 065 Sodiutn lauryl sulfate concentration = 0.10 M. All systems had a quiet discharge. so that the effect upon emulsion stability could be illustrated visually. This is shown in Fig. 3. The sample on the left in Fig. 3 contained no lauryl alcohol and showed complete phase separation. The remaining samples had increasing concentrations of lauryl alcohol, corresponding to the compositions given in Table II. The sample on the extreme right, which had the highest sulfate/alcohol ratio (1:1), showed no phase separation. % ,.•]:.: ...• •' ry.•?•,• :4.- ,,•. . . '.','.':'".:..:•'•'•. •:. ß ß ,::...•r. ::&•q?7'"'. "'. '. Figure 3. Comparative emulsion sta- bilities of sodium lauryl sulfate systems with increasing concentrations of lauryl alcohol (from left to right). The picture was taken four hours after the samples were shakeu An increase in foam stiffness occurred with the first addition of lauryl alcohol, but subsequent additions caused little change. There appeared to be a slight tendency towards increasing density with increasing lauryl alcohol concentrations. Foams with sulfate/alcohol ratios up to 1: • wet paper immediately, but the other foams with higher concentrations of alcohol did not wet
JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS for an hour. The foam with no lauryl alcohol also collapsed soon after discharge, but the remainder of the foams retained their shapes for at least an hour. However, those that wet paper became quite thin on aging as a result of loss of liquid from drainage. In this series, as in many of the series to be discussed later, it is possible to select foams which wet and collapse, or which wet and retain their structure, or are quite stable. Variation in t•ropellants The effect of different propellants upon the properties of sodium lauryl sulfate/lauryl alcohol systems is shown in Table III. Freon-12 and Freon-12/Freon-114 (40/60) gave foams with the lowest drainage rates, and Propellant 152a and Propellant 142b gave foams with the highest. The former two propellants gave the stiffest foams and the latter two the lowest density foams. Wettability data generally correlated with the foam drainage results. The foams with Propellant 152a and Propellant 142b wet paper within five minutes after discharge. The foams with the other propellants did not wet during at least one hour after discharge. The foam with Pro- pellant 152a collapsed within 15 minutes and that with Propellant 142b collapsed within one hour. The other foams retained their shape. Microscopic examination did not reveal any significant differences between foams formulated with Freon-12, Freon-12/Freon-114 (40/60), Freon-114, or Freon-12/Freon-ll (50/50). However, the foams with Propellant 142b or Propellant 152a had much larger laminae that Table III Variation in Propellant--Sodium Lauryl Sulfate•/Lauryl Alcohol Systems b Foam Properties Emulsion Properties Drainage Stiffness Density Type of Propellant Viscosity Stability (2 hr) (g) (g/co) Discharge Freon-12 High 5 hr 20 28 0. 061 Slightly noisy Freon-12/Freon-114 (40/60) High 5 hr 0 44 0. 062 Quiet Freon-114 High 5 hr 0 48 0. 073 Quiet Freon- 12/Freon- 11 (50/50) High 5 hr 2 16 0. 059 Noisy Propellant 142b High 5 hr 41 16 0. 043 Slightly noisy Propellant 152a Medium 5 hr 84 14 0.039 Slightly noisy Sodium lauryl sulfate concentration = 0.10 M. Sulfate/alcohol ratio (molar) = 1:1.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)