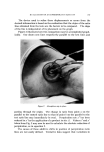
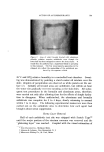
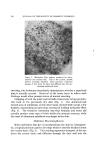
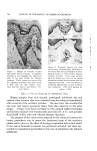
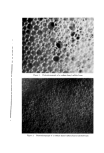
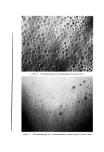
Figure 4. Photomicrograph of a sodium lauryl sulfate/lauryl alcohol foam with Freon- 12/Freon-114 (40/60) propellant .., :.... Figure 5. Photomicrograph of a sodium lauryl sulfate/lauryl alcohol foam with fiuorocarbcm 142b as the propellant
814 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ranged in thickness from about 0.003 to 0.008 in., and the entire system appeared to be very fluid. The foams with the other propellants had a closer packed bubble structure with laminae approximating 0.001 in. in thickness. Microphotographs of the foams prepared with Freon-12/ Freon-114 (40/60) and Propellant 142b are illustrated in Figs. 4 and 5. The photographs show the increase in foam bubble size that occurs with Propellant 142b. The effect of the propellants in systems with sodium lauryl sulfate alone was also investigated. However, in all cases, the foams were very unstable, and no differences due to a variation in propellants were detected. Triethanolamine-Fatty Acid Systems Variation in Alcohols The effect of various alcohols upon the properties of triethanolamine- fatty acid systems is shown in Tables IV, V, VI, and VII. Cholesterol was evaluated separately, and the effect of cholesterol, in comparison with that of the fatty alcohol corresponding to the fatty acid used, is shown in Table VIII. In all of the systems, certain alcohols caused a pronounced increase in emulsion viscosity and stability and a decrease in foam drainage. In these cases, molecular complex formation between the alcohols and the triethanolamine salts was judged to have taken place as a result of the effect of the alcohols upon the properties of the systems. In each triethanolamine-fatty acid system, certain alcohols were more effective than others as far as complex formation with the tri- ethanolamine salt was concerned. The data indicate that there is a slight tendency for the alcohols having about the same number of carbon atoms as that of the fatty acids involved to be the most effective for that particular system. Thus, lauryl alcohol was fairly effective in forming complexes with triethanolamine laurate but not with triethanolamine stearate. Likewise, stearyl alcohol forms strong complexes with tri- ethanolamine stearate but not with triethanolamine laurate. The over-all effects of the alcohols upon the various properties of the triethanolamine-fatty acid systems are summarized in Table IX. This summary should aid in the selection of a triethanolamine-fatty acid system with specific emulsion and foam properties. The effects of the various alcohols upon foam stability varied with the particular system involved, and each system will be discussed separately.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)