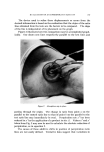
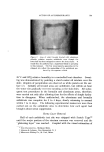
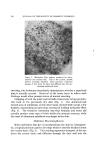
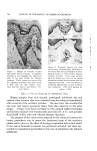
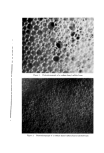
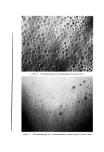
810 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS hols increased foam stiffness. Judging by these results, sodium lauryl sulfate forms complexes with these alcohols in the aerosol systems. Stearyl alcohol, oleyl alcohol, and cholesterol had little effect. The effect of the alcohols upon foam stability was quite marked. Wetting results correlated well with drainage tests. Foams from sodium lauryl sulfate alone, or in combination with oleyl alcohol and cholesterol, caused immediate wetting, followed by that with stearyl alcohol. Foams with lauryl, myristyl, or cetyl alcohol did not wet for at least an hour. The effect upon foam persistence was similar. The foam from so- dium lauryl sulfate alone disappeared completely in 15 minutes, followed shortly thereafter by that with oleyl alcohol and cholesterol. The foam with stearyl alcohol was partially collapsed after one hour, while the remaining foams showed slight thinning but very little other change. The effect of the alcohols upon wettability and foam persistence can be summarized as follows: Foam wettability (in order of increasing wettability) Lauryl alcohol None Myristyl alcohol Stearyl alcohol Oleyl alcohol Cetyl alcohol Cholesterol Foam persistence (in order of decreasing foam persistence) Lauryl alcohol Myristyl alcohol Stearyl alcohol Oleyl alcohol None Cetyl alcohol Cholesterol Microscopic observation of the sodium lauryl sulfate foams showed bubble sizes ranging from about 0.001 to 0.01 in., with laminae thick- nesses of about 0.001 to 0.003 in. The foams with lauryl alcohol present had a smaller bubble size and thinner laminae. The differences between the two foams are illustrated very effectively by the micro- photographs in Figs. 1 and 2. These pictures indicate that complex formation between sodium lauryl sulfate and lauryl alcohol decreases bubble size considerably. Variation in Concentration of Lauryl Alcohol Increasing the concentration of lauryl alcohol in sodium lauryl sul- fate systems increased emulsion stability and decreased foam drainage, as shown in Table II. The samples that were used to obtain the data in Table II were also photographed four hours after they had been shaken
COMPLEX FORMATION IN AEROSOLS Table 11 Variation in Conccntratiou of Lauryl Alcohol in Sodium Lauryl Sulfate Systems 811 Sulfatc"/ Foam Properties •' Alcohol Emulsion Properties Ratio Drainage Stiffness Density (molar) Viscosity Stability (60 rain) (g) (g/cc) 1: 0 Low 1 rain 93 12 0. 056 1: •/• Low 1 min 33 34 0. 059 1: • Low 15-20 min 24 32 O. 062 1: a/• Low 30-60 rain 4 38 O. 063 1:1 Medium 16 hr 0 34 0. 065 Sodiutn lauryl sulfate concentration = 0.10 M. All systems had a quiet discharge. so that the effect upon emulsion stability could be illustrated visually. This is shown in Fig. 3. The sample on the left in Fig. 3 contained no lauryl alcohol and showed complete phase separation. The remaining samples had increasing concentrations of lauryl alcohol, corresponding to the compositions given in Table II. The sample on the extreme right, which had the highest sulfate/alcohol ratio (1:1), showed no phase separation. % ,.•]:.: ...• •' ry.•?•,• :4.- ,,•. . . '.','.':'".:..:•'•'•. •:. ß ß ,::...•r. ::&•q?7'"'. "'. '. Figure 3. Comparative emulsion sta- bilities of sodium lauryl sulfate systems with increasing concentrations of lauryl alcohol (from left to right). The picture was taken four hours after the samples were shakeu An increase in foam stiffness occurred with the first addition of lauryl alcohol, but subsequent additions caused little change. There appeared to be a slight tendency towards increasing density with increasing lauryl alcohol concentrations. Foams with sulfate/alcohol ratios up to 1: • wet paper immediately, but the other foams with higher concentrations of alcohol did not wet
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)