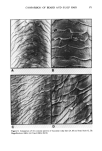
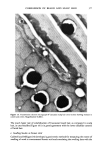
350 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (23) (24) (25) (26) (27) (28.) (29) (30) (31) G. Gillberg, H. Lehtinen, and S. Friberg, NMR and IR investigation of the conditions determining the stability of microemulsions,J. Colloid Interface Sci., 33, 40-53 (1970). E. Sjoblom and S. Friberg, Light-scattering and electron microscopy determination of association structures in W/O microemulsions,J. Colloid Interface Sci., 67, 16-30 (1978). S. Friberg and I. Burasczenska, Microemulsions in the water-potassium oleate-benzene system, Progr. Colloid and Polymer Sci., 63, 1-9 (1978). M.P. Shorr, P. Sharkey, and C. T. Rhodes, Dissolution of hydrocortisone, J. Pharm. Sci., 61, 1732-1735 (1972). B. W. Barry and D.I.D. E1 Eini, Solubilization of hydrocortisone, dexamethasone, testosterone and progresterone by long chain polyoxyethylene surfactants.J. Pharm. Pharmac., 28, 210-218 (1976). B. R. Hajratwala and H. Taylor, Effect of non-ionic surfactants on the dissolution and solubility of hydrocortisone,J. Pharm. Pharmac., 28, 934-935 (1976). A. E. Allen and V. D. Gupta, Stability of hydrocortisone in polyethylene glycol ointment base, J. Pharm. Sci., 63, 107-109 (1974). V. K. Bansal, D. O. Shah, and J.P. O'Connell, Influence of alkyl chain length compatibility on microemulsion structure and solubilization,J. Colloid Interface Sci., 7 5,462-475 (1980). K. E. Bennett, J. C. Hatfield, H. T. Davis, C. W. Macosko, and L. E. Scriven, Viscosity and Conductivity of Microemulsions, in Microemulsions, et aL, ed. I.D. Robb (Plenum Press, New York, 1982), pp 65-81. (32) J. P. Carey et al, The Rheology of Crude Oil Dispersions, SPE paper 5299, Society of Petroleum Engineers of AIME, 1975. (33) M. Lagues and C. Sautery, Percolation transition in water in oil microemulsions, electrical conductivity measurements,J. Phys. Chem., 84, 3503-3508 (1980). (34) J. Lagourette, J. Peyrelasse, C. Boned, and M. Clausse, Percolative condition in microemulsion type systems, Nature, 281, 60-62 (1979). (35) S. H. Yalkowsky and T.J. Roseman, in "Techniques of Solubilization of Drugs," Drugs and the Pharmaceutical Sciences, Vol. 12, ed. S. H. Yalkowsky (Marcel Dekker, Inc., 1981), p 129. (36) D. O. Shah and R. S. Schechter, eds., Improved Oil Recovery by Surfactant and Polymer Flooding (Academic Press, New York, 1977). (37) D. O. Shah, ed., Surface Phenomena in Enhanced Oil Recovery (Plenum Press, New York, 1981). (39) D. O. Shah, The world of surface science, Chem. Eng. Education, Winter 1977, 14-48. (39) S. I. Chou and D. O. Shah, The effect of H20 and D20 on colloidal properties of surfactant solutions and microemulsions,J. Colloid Interface Sci., 80, 49-54 (1981). (40) C. Ramachandran, S. Vijayan, and D. O. Shah, Effect of salt on the structure of middle phase microemulsions using spin label technique,J. Phys. Chem., 84, 1561-1567 (1980). (41) S. I. Chou and D. O. Shah, Dielectric relaxation of external microemulsions, J. Phys. Chem., 85, 1480-1485 (1981). (42) D. O. Shah, in "Proceedings of the European Symposium on Enhanced Oil Recovery" (Elsevier Sequoia, S.A., 1981), pp 1-40. (43) D. O. Shah, High resolution NMR studies on the structure of water in microemulsions and liquid crystals, Ann. New York Acad. Sci., 204, 125-133 (1973).
j. Soc. Cosmet. Chem., 34, 351-359 (November 1983) Application of a new microbiological technique to the study of antiperspirant and deodorant soap efficacy R. F. THEILER, C. L. SCHMIT, andJ. R. ROHEIM, Armour Research Center, New Science and Technology Department, 15101 N. Scottsdale Road, Scottsdale, AZ 85260. Received January 10, 1983. Synopsis A new technique for sampling cutaneous microorganisms has been used to study the effects of deodorant products on aerobic axillary microbial populations. Initially the Thran spray gun was compared to the mechanical scrub method and was found to compare favorably in terms of reproducibility and sensitivity. When a commercial stick antiperspirant was evaluated, both methods demonstrated similar log reductions in axillary bacteria when compared to controls. The Thran spray gun was also used to study the efficacy of a commercial deodorant soap containing 3,4,4'-trichlorocarbanilide. A 55% reduction in aerobic axillary microorganisms was demonstrated 24 hours after washing with the soap. INTRODUCTION Many locations on the surface of human skin emit distinctly different odors. However, of all the human scents, those emanating from the axilla are regarded by society as some of the most offensive. In recent years the accumulation of a significant body of scientific evidence has traced the source of axillary odors to the action of bacteria on secretions from the apocrine gland (!-4). In a number of recent publications it has been suggested that a class of Gram positive microorganisms, the diphtheroids, are responsible for the selective generation of the distinctly pungent axillary odors, while the micrococci are responsible for the generation of sweaty, acid odors (2-4). Therefore, numerous manufacturers of deodorant products have marketed formula- tions with an active ingredient which reduces axillary microbial populations for the purpose of reducing the intensity of axillary odor. Since bacterial reduction is related to a product's deodorant efficacy, numerous methods have been developed for qualitatively and quantitatively monitoring the distribution and population of skin flora. Among these are mechanical scrub techniques (5-6), swabbing procedures (7-9), tape stripping and contact plates (7-13), as well as basin scrubbing methods (14-15). Among these techniques, a widely employed procedure for quantitating aerobic skin micro flora has been the mechanical scrub technique which involves a timed scrub of the skin surface with a blunt teflon scrubber 351
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)