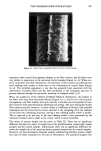
136 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS rials are especially critical because traces of NOx adsorbed to these sorbents lead to nitrosamine formation. Removal of the amine by adsorption to a cation exchange resin prevents artifact formation. Subsequent clean-up on a silica gel 40-column represents an efficient further purification step. Analysis of high purity DEA by this method did not show detectable NDELA contamination (5 ppb), which proves that artifact for- mation is inhibited. For trimethylsilylation, the conditions described were found to be appropriate heating in a closed system to 80øC did not improve the yields. A gas chromatogram as obtained from a typical diethanolamine sample is illustrated in Figure 1. Baseline separation of internal standard (NEHPA-TMS) and contaminating nitrosamine (NDELA-TMS) is obtained. To test the reproducibility of the analytical procedure, a sample of DEA was analyzed ten times. An average NDELA content of 279 }xg/kg _ 11 }xg/kg (SD = standard deviation) was found. The average recovery of the internal standard was 89 - 8% (SD). The determination limit was 5 }xg/kg in our hands. Preliminary results of a first collab- orative study indicate that this limit might not be reached in all instances, depending on analytical experience and sensitivity of the Thermal Energy Analyzer. Therefore, a range of 5- 15 }xg/kg is given. As can be seen from Table I, most of the alkanolamines analyzed are contaminated with the corresponding nitrosamines. This is in agreement with results obtained earlier with a series of secondary alkylamines (14). The finding that specific samples showed no detectable contaminations demonstrates that nitrosamine formation obviously can be reduced or prevented by maintaining appropriate conditions for production and/or storage of the corresponding amines. To examine possibilities for removal of nitrosa- mine contamination, DEA contaminated with 280 ppb NDELA was treated overnight with Ni/A1 alloy (0.5 g of the amine, diluted in 10 ml water, 150 mg of alloy). This treatment resulted in a 90% reduction of the NDELA content. ACKNOWLEDGMENTS This study was supported by the German Ministry for Youth, Family, Women and Health (Kap. 1502, title 53201). We thank Miss B. Weber for competent technical assistance. Table I Contamination of Alkanolamines With NDELA and NBHPA NDELA (•g/kg) NBHPA (•g/kg) DEA 1 DEA 2 DEA 3 DEA 4 TEA MEA 1 BHPA 1 BHPA 2 BHPA 3 91 1460 279 5 5 41 465 36 13
NITROSOALKANOLAMINE DETERMINATION 137 REFERENCES (1) P. Pour, F. W. Kriiger, J. Althoff, A. Cardesa, and U. Mohr, Effect of beta-oxidized nitrosamines in syrian golden hamsters, J. Natl. Cancer Inst., 54, 141-145 (1975). (2) J. Hillrich, J. Schemltz, and D. Hoffman, Tobacco carcinogenesis 17. Effects of N-nitrosodiethano- lamine and 1,1-diethylhydryzine in syrian golden hamsters, Cancer Lett., 4, 55-60 (1977). (3) P. Pour, S. Salmasi, R. Runge, R. Gingell, L. Wallcave, D. Nagel, and K. Stepan, Carcinogenicity of N-nitroso-bis(2-hydroxypropyl)amine and N-nitroso-bis(2-oxopropyl) amine in MCR rats, J. Natl. Cancer Inst., 63, 181-190 (1979). (4) R. Preussmann, M. Hahs, H. Hahs, and D. Schmiihl, Carcinogenicity of N-nitrosodiethanolamine in rats at five different dose levels, Cancer Res., 42, 5167-5172 (1982). (5) D. Hoffmann, A. Rivenson, J. D. Adams, A. Juchatz, N. Vinchkoski, and S.S. Hecht, Effects of route of administration and dose on the carcinogenicity of N-nitrosodiethanolamine in the syrian golden hamster, Cancer Res., 43, 2521-2524 (1983). (6) W. Lijinski and M. D. Reuber, Dose-response study with N-nitrosodiethanolamine in F 344 rats, ?d. Chem. Toxic, 22, 22-26 (1984). (7) T. Y. Fan, U. Golf, L. Long, D. H. Fine, G. P. Arsenault, and K. Biemann, N-nitrosodiethanola- mine in cosmetics, lotions and shampoos. ?d. Cosmet. Toxicol., 15, 423-430 (1977). (8) D. Klein, A.M. Girard, J. DeSmerit, Y. Fellion, and G. Debry. Analyse de la nitrosodiethanolamine dans les produits de l'industrie cosmetique. ?d. Cosmet. Toxicol., 19, 233-235 (1981). (9) B. Spiegelhalder and R. Preussmann, Contamination of toiletries and cosmetic products with volatile and nonvolative N-nitroso carcinogens, J. Cancer Res. Clin. Oncol., 108, 160-163 (1984). (10) H. Sommer and G. Eisenbrand, A method for the determination of N-nitrosoalkanolamines in cos- metics, Z. Lebensm. Unters. Forsch., 186, 235-238 (1988). (11) Industrieverband K/Srperpflege und Waschmittel e.V., Empfehlung zur Vermeidung von Nitrosa- minen, Okt. 1983, Frankfurt. (12) Bundesgesundheitsamt empfiehlt )•nderung der Zusammensetzung bestimmter Kosmetika, Bundesge- sundheitsblatt, 30, 114 (1978). (13) G. Eisenbrand, G. Ellen, R. Preussmann, P. L. Schuller, B. Spiegelhalder, R. W. Stephany, and K. S. Webb, "Determination of Volatile Nitrosamines in Food, Animal Food and Other Biological Materials by Low-Temperature Vacuum Distillation and Chemiluminescence Detection," in N-Nitroso Compounds, H. Egan, R. Preussmann, J. K. O'Neill, G. Eisenbrand, B. Spielgelhalder, M. Casteg- naro, and H. Bartsch, Eds. (IARC Sci. Publ., Lyon), Vol VI, pp. 181-203. (14) B. Spiegelhalder, G. Eisenbrand, and R. Preussmann, Verunreinigungen von Aminen mit N-Nitro- saminen, Angew. Chem., 90, 379-380 (1978).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)