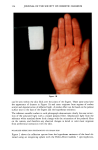
EMULSION VEHICLES AND VASOCONSTRICTOR ACTIVITY 145 dQ AKmDC o - (1) dt h where dQ/dt is the flux h is the thickness of the skin Q, the amount of drug diffused into the receptor fluid at time t D, the diffusion coefficient of the drug in the skin and A, the area of the drug applied on the skin. Equation (1) can be rearranged to, dQ/dt _KmD (2) Kp -- C O A h where Kp is the mean permeability constant. IN VIVO VASOCONSTRICTOR STUDIES The vasoconstructor activity of hydrocortisone 17-valerate 0.2% in the finally selected emulsion was evaluated clinically and compared to that of other marketed corticosteroid products in healthy human volunteers. The method used was similar to the vasocon- striction assay described by Burdick eta[. (16), which is a modification of the bioassay described by McKenzie and Stoughton (17). Approximately 3 mg of each preparation was applied to 12-16 sites (each 7 mm x 7 mm in size) on the volar surface of the forearms of 24 normal healthy subjects. The materials remained on the skin for six hours, unoccluded but protected from mechanical abrasion by an elevated plastic arm guard. A restricted randomization process, balanced for sites and arms, was used in assignment of preparations to the sites in groups of 8 or 12 subjects. One or two pairs of observers independently evaluated the blanching re- sponses, on a scale of 0 to 3, at 7, 9, 11, 13, and 24 hr after application of the materials, in three separate assays. The results of the vasoconstrictor studies were as- sayed by using the area under the time-response curves according to the trapezoidal rule. The data were evaluated by analysis of variance, using Duncan's procedure for multiple comparisons with p • 0.05 for adjacent pairs of means. RESULTS AND DISCUSSION FORMULATION EVALUATION Thirty-six hydrocortisone 17-valerate 0.2% o/w emulsions (see Table I) were studied. The three major ingredients -- petrolatum, mineral oil, and water--which most likely affect the occlusivity of the emulsion system were treated as variables in the formulation study while other ingredients were held constant. Preparation of formulations with low water content (8.9% and 18.9%) was found to be difficult, presumably because the amount of emulsifiers in these formulations was insufficient for the high oil content formulas. The combination of three variables gives different phase-volume ratios for formulations (see Table I). PHYSICAL STABILITY EVALUATION Based upon the scoring system (see Table II), it was observed that formulas with low water content (4) = 3.59) were not physically stable for three weeks at 55øC, ! month
146 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS at 45øC, and two months at 35øC. Although formula #8 seemed to have better physical stability after two months at different temperatures, it still started to bleed within three months. Formulas containing 18.9% water were physically stable for at least four months. The physical instability of these formulas was likely due to incomplete emulsi- fication observed during the preparation of these formulas. Formulas containing 28.9% or more water were found to be physically stable for at least four months at all storage temperatures. CONSISTENCY DETERMINATION Most of the emulsions prepared in the study were highly viscous, and their viscosity and yield values were difficult to measure with a conventional viscometer. A penetrometer was used to measure the consistency of the emulsions. The consistency results are plotted in Figure 1. As can be seen, at constant concentration of petrolatum (or mineral oil), the formulas become high-consistency emulsions as the concentration of mineral oil (or petrolatum) is increased. However, regardless of the water concentration, as soon as the total concentration of the oil phase reaches 60% or more, slight decreases in consistency (slight increases in penetration depth) were observed. This phenomenon might be due to insufficient emulsification power, where the unemulsified oil phase acts as a lubricant causing deeper penetration of the plunger. VOLATILITY DETERMINATION Figure 2 represents the data obtained from water evaporation rates determined by an evaporimeter and plotted against the concentration of mineral oil in the formulas. The phase-volume ratio indicated in parentheses also follows the trend in which the water evaporation rate decreases as the phase-volume ratio increases. At a constant concentra- tion of mineral oil, the water evaporation rate decreases inversely with the concentration of petrolatum but is directly proportional to the concentration of water in the formulas. The trend presented in Figure 2 seemed to agree reasonably well with the results of consistency in Figure 1 however, the water evaporation rate decreased continuously even at low water content level (8.9% and 18.9%), whereas the consistency of the same formulas increased slightly. Due to the limited source of excised human skin, a small group of formulas was selected for further evaluation. The selection was based on physicochemical stability results and water evaporation rates which suggest that at least 28.9% water might be needed to achieve complete emulsification and at least 40% oil phase is needed to reduce the water evaporation rate. A "one-factor-at-a-time" technique was adopted for formulation opti- mization. The approach used was to select a center formula (#28) which meets the above two criteria. Two straight lines were drawn perpendicularly across formula #28 as shown in Figure 2 (dotted line). Formulas (#2, # 10, # 17, #23, and #28--desig- nated as group 1) selected from the vertical line, which represents formulations with different phase-volume ratios from 0.19 to 1.34, were studied first to determine the effect of the o/w phase-volume ratio. Secondly, at a fixed 4) (1.34) value, formulas (#6, #13, #19, #24, #31, and #28--designated as group 2) from the horizontal line representing formulations with different petrolatum content, were studied for any ac- tivity increase that could be further enhanced by increasing the concentration of petro-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)