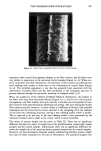
EMULSION VEHICLES AND VASOCONSTRICTOR ACTIVITY 153 4 % Formule # Petrolatum [] 28 40 o $t 50 & t9 20 [] 24 50 + 6 0 ß t3 t0 zx 36 70 0 4 8 2:4 2:8:32: 48 52: 56 72: Time, hours Figure 6. In vitro human skin permeation profiles of the selected hydrocortisone 17-valerate 0.2% emul- sions with 4) = 1.34. The bar indicates the standard error of the means (n = 3). choice for hydrocortisone 17-valerate. Formula #28 was then included in the compara- tive efficacy and safety evaluations against some marketed corticosteroid products. The degree of vasoconstriction induced by HCV 0.2% in formula #28 and a marketed cream, relative to that of other corticosteroid products, was evaluated by Sefton et al. (1) as shown in Table V. The results indicated that 0.2% HCV in both formula #28 and cream is associated with equal or greater vasoconstrictor responses than are other corti- costeroid preparations of intermediate or moderate potency corticosteroids, including formulations of triamcinolone acetonide 0.1% cream, fluocinonide 0.05% cream and ointment, and betamethasone 17-valerate 0.1% cream. It was also noted that formula #28 induces statistically greater vasoconstriction (by Duncan's procedure) than does HCV cream at 0.2% level. In order to examine the relationship between vasoconstrictor activity and skin perme- ation rate, an additional in vitro skin permeation study on the marketed HCV 0.2% cream was performed. According to the formulation information (7,8), the HCV 0.2% cream has a phase-volume ratio of 0.61. The resulting data from the skin permeation
154 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table IV Permeability Constants (K v) of the Selected 12 Hydrocortisone 17-Valerate 0.2% o/w Emulsions (n = 3) Formula no. Kp, cm/hr 1 3.20 X 10-5(3.97 x 10-6) * 0.06 2 2.40 x 10 -5 (8.66 x 10 -7) 0.19 6 4.34 X 10-5(5.54 X 10 -6 ) 1.34 10 3.57 X 10-5(9.01 X 10 -6 ) 0.35 13 4.14 X 10 -5 (1.85 X 10 -6 ) 1.34 17 3.08 X 10-5(4.89 X 10 -6 ) 0.57 19 5.11 X 10-5(3.03 X 10 -7 ) 1.34 23 3.56 X 10-5(7.10 X 10 -6 ) 0.88 24 4.78 X 10-5(3.85 X 10 -6 ) 1.34 28 5.71 X 10-5(1.05 X 10 -7 ) 1.34 31 5.46 X 10-5(8.31 X 10 -7 ) 1.34 36 3.10 X 10-5(3.65 X 10 -6 ) 3.59 * Standard error of the means (n = 3). study indicate that the permeability constant 3.50 x 10 -5 cm/hr (s.e. = 5.10 x 10 -7 cm/hr) of the marketed HCV 0.2% cream was 0.6 times that of 5.71 X 10 -5 cm/hr (s.e. = 1.05 x 10 -7 cm/hr) of formula #28. Correspondence between the in vitro skin permeation rate and the in vivo vasoconstrictor activity is indicated in this study. It is therefore reasonable to assume that the enhanced vasoconstrictor activity of formula #28 can be attributed to its higher occlusivity. i Y= 2__.0:35 X 10 -5 + 2.446 X lO -5 X r: 0.902:1 0 0.25 0,50 0,75 1.00 1.25 1.50 1.75 Oi[/W(]ter Ph(]se-Vo[ume R(]tio (•) Figure 7. Correlation of the permeability constants and phase-volume ratios (4)) of the selected o/w emul- sions. The bar indicates the standard error of the means (n = 3).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)