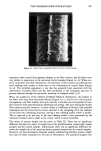
EMULSION VEHICLES AND VASOCONSTRICTOR ACTIVITY 15 5 6 o#28 #3i 5 m. t#13 #i9Q #24 Y=4.24 x iO -5 + 2.77 x 10 -7 (r= 0.8514) o lo 20 30 40 50 x % Petrotatum = 34) Figure 8. Linear correlation of permeability constants and % of petrolatum of the selected o/w emulsions with 4) = 1.34. The bar indicates the standard error of the means (n = 3). CONCLUSIONS In conclusion, the findings of this study suggest that the occlusivity of an o/w emulsion system can be enhanced by adjusting its o/w phase-volume ratio with petrolatum. This enhanced occlusivity will, in turn, promote the in vitro human skin permeation rate. However, occlusivity provided by too high a concentration of petrolatum did not en- hance the permeation of HCV. This study shows that petrolatum, not mineral oil, is the key ingredient providing the occlusivity of the o/w emulsion system. In vivo vaso-
156 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table V Vasoconstrictor Activity of Various Corticosteroid Creams and Ointments in Relation to That of Hydrocortisone 17-Valerate 0.2% Cream (assigned the value of 1.00) Mean activity Formulation (n -- 24) Fluocinonide 0.05 % ointment (Lidex, Syntex Lab Inc., Palo Alto, CA) Fluocinonide 0.05 % cream (Lidex, Syntex Lab Inc., Palo Alto, CA) Desoximetasone 0.25% cream (Topicort, Hoechst-Roussel Pharm. Inc., Somerville, NJ) Hydrocortisone Valerate 0.2% emulsion (Formula #28, later designated HCV 0.2% ointment) (Westcort, Westwood Pharm. Inc., Buffalo, NY) Desoximetasone 0.05% cream (Topicort, Hoechst-Roussel Pharm. Inc., Somerville, NJ) Hydrocortisone Valerate 0.2% cream (Westcort, Westwood Pharm. Inc., Buffalo, NY) Betamethasone Valerate 0.1% cream (Valisone, Schering Corp., Kenilworth, NJ) Betamethasone Valerate 0.01% cream (Valisone, Schering Corp., Kenilworth, NJ) Triamcinolone Acetonide 0.1% cream (Kenalog, E. R. Squibb & Sons Inc., Princeton, NJ) 1.5 P* 1.27 b 1.22 c 1.11 d 1.07 d 1.00 e 0.47 f 0.46 f 0.32 g * For each assay, means identified with different letters (i.e., a-g) are significantly different (P • 0.05 for adjacent means) from each other by Duncan's procedure. Different sets of formulations were tested in each assay. (Courtesy of Sefton et al. (1).) constrictor activity studies further support the in vitro findings, where formula #28 has a higher skin permeation rate as well as a higher vasoconstrictor activity than that of the HCV 0.2% cream, which could be due to the occlusivity of the vehicle. ACKNOWLEDGMENTS The authors are grateful to Mr. G. Meyerborer and Mr. C. Dahlheim for their excellent technical assistance during the study. REFERENCES (1) J. Sefton, J. s. Loder, and A. A. Kyriakopoulos, Clinical evaluation of hydrocortisone 17-valerate 0.2% ointment, Clin. Ther., 6(3), 282-293 (1984). (2) J. Ziegenmeyer, "The Influence of the Vehicle on the Absorption and Permeation of Drugs," in Dermal and Transdermal absorption, R. Brandau and B. H. Lippold, Eds. (Wissenschaftliche Verlags- gesellschaft GmbH, Stuttgart, Munich, 1981), pp. 73-86. (3) M. Katz and B. J. Poulsen, Corticoid, vehicle, and skin interaction in percutaneous absorption, J. Soc. Cosmet. Chem., 23,565-590 (1972). (4) D. D. Munro, The relationship between percutaneous absorption and stratum corneum retention, Br. J. Dermatol., 81(suppl. 4), 92-97 (1969). (5) B. Idson, Dermatological emulsions, Cosmet. Toil., 95, 59-62 (1980). (6) R. C. Cornell and E. B. Stoughton, Correlation of the vasoconstriction assay and clinical activity in psoriasis, Arch. Dermatol., 121, 63-67 (1985).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)