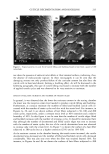
EFFECT OF CURLING IRONS 229 1.2 0.8 0.6 0.4 0.2 -0.2 30s ......... 15s \ 0.003s % 0.0009s 2s 0.2S 0 2 4 6 8 10 12 Figure 2. Dimensionless temperature response in a semi-infinite solid (superpositioned fiber assembly) exposed to a high-temperature surface. The largest value of the distance parameter (10.69) corresponds to the fiber assembly thickness (1750 pm). Also, the calculations for the temperature distribution throughout the fiber assembly were performed for exposure times ranging from 0.0009 s to 30 s. The calculated data presented in Figure 2 clearly demonstrate that near-uniform temperature distributions in hair samples are reached within a few seconds of thermal exposure. Under real conditions, the uniform temperature distributions are probably reached even
230 JOURNAL OF COSMETIC SCIENCE faster, since hair is heated from both sides of the curling iron assembly. The results of the theoretical heat-transfer calculations suggest that the thermal conditions of continu- ous and intermittent treatment modes are nearly equivalent and should lead to similar degrees of fiber damage. FLUORESCENCE ANALYSIS Figure 3 presents the fluorescence spectra of white, unpigmented hair, subjected to thermal treatment at 164øC for various periods of time ranging from 0 to 30 min. The spectra were obtained using excitation wavelengths of 290, 320, and 350 nm and show emission bands at 345,420, and 465 nm. The emission band, with a maximum at 345 nm, was previously shown to correspond to Trp, which absorbs light with a maximum at 285 nm (14). The peak at 465 nm, obtained by excitation at 320 and 350 nm, matches the emission band of l-kynurenine, which has an absorption maximum at approximately 360 nm (26,27). Evident from the spectra is a peak at 465 nm, obtained by excitation at 290 nm, which is probably related to disulfide bonds in the keratin structure (26). The emission maximum at 420 nm can be ascribed to N- formylkynurenine, which according to the literature has an absorption maximum at 320 nm (27). The spectra obtained after thermal exposure indicate a decrease in the emission inten- sities of all bands, which is probably related to thermal decomposition of the corre- sponding chromophores. The largest reduction in the emission intensity is evident for the band at 345 nm, corresponding to Trp. Figure 4 presents the time dependence of Trp decomposition for various types of hair, including unpigmented (white), Piedmont, commercially bleached, light-brown, and Asian hair. The values of % Trp were calcu- lated as the ratios of Trp emission intensities obtained before and after the thermal exposure (% Trp = It/I ,, where I t and I, represent the Trp emission intensities of the thermally exposed and unexposed regions of a hair tress, respectively). All types of fibers exhibit rapid Trp decomposition, with only 20% of the Trp residues remaining after 30 min of thermal exposure. Further analysis of the data has also shown that the process of Trp decomposition follows first-order reaction kinetics for which the calculated rate constants are given in Figure 4. The temperature effect on the extent of Trp decompo- sition is illustrated with the data presented in Figure 5, which were obtained after a total treatment time of 3 min. The results include measurements obtained using intermittent and continuous modes of treatment. As predicted by the heat-transfer calculations, which showed nearly uniform temperature distributions within a few seconds of expo- sure time, both intermittent and continuous modes of hair treatment yielded similar (within experimental error) extents of Trp loss in the temperature range of 140øC to 165øC. As stated previously, one side of a hair tress was exposed to the high-temperature surface of the curling iron while the other side of a tress was in contact with the lower-temperature arm. We found that both sides of the hair tresses experienced a comparable degree of Trp decomposition. A high rate of heat transfer is presumably responsible for the similar degrees of Trp decomposition on both sides of a tress. The data presented in Figure 5 also demonstrate that the process of Trp decomposition is thermally activated with an estimated activation energy of 6.6 kcal/mol. Such a small value of activation energy suggests a free radical mechanism for the oxidation of Trp (28•.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)