PERCUTANEOUS ABSORPTION OF LACTIC ACID 259 EXPERIMENTAL MATERIALS L+[•4C(u)] lactic acid, specific activity 150 mCi/mmol, was obtained from American Radiolabelled Chemicals Inc. The following chemicals were used in formulation of the emulsions: a hydrophilic surfactant (Synperonic PE/F127, a block copolymer of ethylene oxide and propylene oxide ICI Surfactants, Wilmington, DE) a lipophilic surfactant (Hypermer A60, a modified polyester ICI Surfactants) lactic acid (USP grade Purac) paraffin oil (Penreco) and propylene glycol (Fischer Chemical). The scintillation cock- tails used were Ecolume TM (ICN), Scintiverse7 ScintanalyzerTM (Fischer Chemicals), and NCS-II-Tissue solubilizer (Amersham Canada Limited). PREPARATION OF EMULSIONS All emulsions were prepared from the same formula: paraffin oil 35% w/w, Hypermer A60 2.8% w/w, Synperonic 1.2% w/w, lactic acid 8% w/w, pH adjuster KOH, and balance water. The hydrophilic lipophilic balance (HLB) (16) for Synperonic, was ap- proximately 20, and that of Hypermer was between 2 and 4. The calculated HLB of the surfactant mixture was 9.0. The radiochemical concentration of lactic acid in all the emulsions was 30 pCi/g. The emulsions were stored at 4øC overnight before use in the experiments. The simple emulsions (o/w or w/o) were prepared by adding the aqueous phase, con- taining unlabeled lactic acid and the L-[14C(u)] lactic acid, to the oil phase at 65ø-70øC. For the o/w emulsion, the hydrophilic surfactant was in the aqueous phase and the lipophilic surfactant was incorporated in the oil phase, whereas for the w/o emulsion both the surfactants were in the oil phase. A coarse emulsion was first formed by mixing the two phases at 65ø-70øC in a Tekmar RW 20 DZM mixer for fifteen minutes at 1500 rpm. The emulsion was then homogenized for five minutes with the Silverson L4R homogenizer. Although the bulk compositions were the same, the interfacial compositions varied depending on the formulation procedure. The adsorption of surfactant monomers at the oil-water interface involves dissociation of the surfactant aggregates in the bulk phase to monomers followed by diffusion of the monomers to the interface. The more soluble the surfactant is in the bulk phase, the faster are the aggregate-monomer breakdown kinet- ics. When the hydrophilic and hydrophobic surfactants are in the water and oil phases, respectively, the aggregate-monomer breakdown kinetics is high for both of them. As a result, both the hydrophilic and hydrophobic monomers adsorb at the oil-water interface during the emulsification process. The HLB of the surfactant mixture at the interface in that situation is quite similar to the HLB of the total system. For the surfactant system (HLB of 9) chosen in this study, such an interface stabilizes an o/w emulsion. However, when the hydrophilic surfactant is dispersed in the oil phase, the aggregate-monomer dissociation kinetics are slow, and consequently only a small amount of the monomer reaches the oil-water interface during the emulsification process. The interface in this situation predominantly contains the low-HLB hydrophobic surfactant that stabilizes a w/o emulsion.
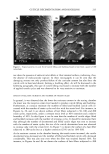
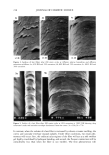
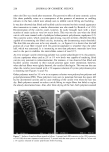
260 JOURNAL OF COSMETIC SCIENCE The w/o/w multiple emulsion was prepared by a two-stage emulsification procedure. In the first step, the w/o primary emulsion was formed at 75øC by adding the aqueous phase, containing unlabeled and labeled lactic acid, to the oil phase containing the hydrophobic surfactant. In the second step, the w/o primary emulsion was dispersed at 50øC in an aqueous solution containing the hydrophilic emulsifier. In this case, two separate oil-water interfaces were created, an inner one with the hydrophobic surfactant and an outer one with the hydrophilic surfactant. EMULSION CHARACTERIZATION The emulsions were characterized using the light microscope at a magnification of 100 (Zeiss M 80, Germany). The emulsion drop size was determined by the Malvern Mas- tersizer using the Mie theory of light scattering. The o/w emulsion was distinguished from the w/o emulsion using the water- and oil-soluble dyes methylene blue and Sudan IV, respectively. The characteristic parameters of the emulsions are shown in Table I. The microscopic aspect of the w/o/w multiple emulsion was characteristic of these systems. The mean diameter of the multiple oil globules observed in the w/o/w emul- sions was determined to be 155 l•m. The globules of the o/w simple emulsion showed a mean diameter of less than 30 •m, and the w/o globules had a mean diameter of less than 35 l•m. SKIN SAMPLES The in vitro percutaneous absorption measurements were carried out with 3-4-week-old female porcine dorsal skin obtained from Buckshire Corp. (Perkasie, PA) and stored at -75øC. The skin was thawed, and adipose tissue and hair were removed. The shaved skin was then dermatomed to 510-1•m thickness using a Padgett Dermatome, and 13-ram discs were cut from the dermatomed skin and mounted on Bronaugh flow-through cells (14). After each skin disc was equilibrated for 30 minutes, transepidermal water loss (TEWL) measurements were carried out using an evaporimeter (Servo-Med AB, Stock- holm, Sweden) to check its barrier integrity. Skins with TEWL values greater than 5 gm/cm2/hour were rejected. TEWL measurements were also carried out to monitor the change in skin hydration after product application. PERCUTANEOUS ABSORPTION MEASUREMENTS The experiments were carried out at two dose levels, 2-1•1 topical film (finite dose) or 75-1•1 "infinite" dose applied on a 0.64 cm 2 skin disc. The product was spread evenly Table I Characteristics of the O/W, W/O, and W/O/W Emulsions Continuous Emulsion Microscopic aspect phase O/W simple emulsion W/O simple emulsion W/O/W multiple emulsion Simple globules 25-30 Simple globules 30-35 Multiple globules 150-160 •rn Aqueous Oily Aqueous
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)