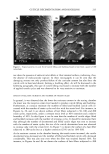
PERCUTANEOUS ABSORPTION OF LACTIC ACID 267 Table II Effect of pH on the Epidermal and Dermal Concentration of Lactic Acid Six Hours After In Vitro Application of an Oil-inWater Emulsion Mode of application pH Epidermis (mM) Dermis (mM) 2plTopicalfilm 3.8 24.9ñ3.5* 1.6ñ0.3 7.0 4.6ñ1.1 1.1ñ0.1 75plOccludedpatch 3.8 23.1ñ5.6 14.1ñ4.5 7.0 20.4ñ4.5 5.4ñ2.2 * Standard error of the mean (SEM). j=PAC (1) where KD p _ (2) L In the above equations, AC is the difference in the active concentrations between the donor and the receptor sides, K is the distribution coefficient of the active between the top layer of the corneum and the donor phase, and D is the diffusivity of the active in the corneum of thickness L. From Figure 4, the initial receptor flux was 0.16 pg/cm2/hr, 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 • 75 gl o/w with PG •e l* {--I 75 gl o/w without PG pO.01 T 8O 0.0 0 Corneum Epidermis Dermis Tissue deposition Figure 6. Tissue deposition of lactic acid from a 75-1•1 infinite dose o/w emulsion at pH 3.8 with (5%) or without propylene glycol. Tissue concentrations (n = 6) were measured six hours after application. Error bars represent SEM. The cumulative receptor penetration at six hours in the presence of propylene glycol is 0.2% ñ 0.0 SEM and that in the abscence of propylene glycol is 0.1% ñ 0.0 SEM. 70 6O • 5O m 4O • 30 20 lO '
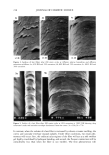
268 JOURNAL OF COSMETIC SCIENCE 25 • 20 • 15 • 10 u. 5 50 40 '• 30 20 m 10 E • 2 gl o/w film with PG [--1 2 gl o/w film without PG ** pO.01 o o Corneum Epidermis Dermis Tissue deposition Figure 7. Tissue deposition of lactic acid from a 2-1•1 finite-dose o/w film at pH 3.8 with (5%) or without propylene glycol. Tissue concentrations (n = 6) were measured six hours after application. Error bars represent SEM. The cumulative receptor penetration at six hours in the presence of propylene glycol is 0.7 + 0.3 SEM and that in the absence of propylene glycol is 0.05 + 0.0 SEM. and the aqueous concentration of lactic acid in the donor side was 0.1778 gm/ml (equivalent to 8% concentration of lactic acid in the total emulsion). The initial per- meability coefficient of lactic acid corresponding to these values was calculated as 8.9 x 10 7 cm/hr. As mentioned earlier, finite-dose receptor fluxes at pH 3.8 and 7.0 (Figure 4) were not significantly different. Receptor fluxes for glycolic acid were found to be pH-sensitive in a 24-hour finite-dose study (13). The lack of pH sensitivity seen in the present study is possibly due to the much shorter (six hours) duration. As the permeability of lactic acid in skin is relatively low, only a very small fraction of the applied dose will reach the systemic circulation in six hours. It is possible that the observed receptor flux in the present study was predominantly due to diffusion through an aqueous shunt or ap- pendagal pathways and hence did not depend significantly on the pH of the vehicle. The lack of dependance of lactic acid penetration on pH, in the infinite-dose situation, suggests that the skin does not act as a lipophilic barrier in that situation. Here, in contrast to the finite-dose situation, the SC remained hydrated during the course of the study. It has been postulated (18,21) that hydrophilic actives can go through hydrated corneum through aqueous pores or channels and that transport through the water-filled pores is independent of the state of ionization as well as of the oil-water partition coefficient. The transdermal permeability coefficient for lactic acid was significantly higher in the infinite-dose application. From the steady-state slope of the infinite-dose situation (Fig- ure 5), a flux of 1.21 pg/cm2/hr was calculated. The corresponding permeability coef-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)