PERCUTANEOUS ABSORPTION OF LACTIC ACID 261 over the entire surface using an applicator. The cells were covered with parafilm in the case of infinite dose to avoid evaporation of the vehicle. Phosphate-buffered saline at pH 7.4 was used as the receptor fluid. The flow rate was controlled at 5 ml/hr. The receptor fluids were sampled every half hour for six hours after dosing the skins. At the end of six hours each flow cell was washed with deionized distilled water three times to remove the excess formulation from the surface. In the case of the infinite-dose application, the excess emulsion was removed with a cotton swab before washing the skin. The skin was dismounted from the cell and carefully swabbed with a Kimwipe TM. The stratum corneum was obtained from the skin disc using nine tape strippings (3M Scotch Magic TM Tape). TEWL measurements indicated that the corneum barrier was com- pletely removed by nine tape strippings. The epidermis was then scraped from the dermis using a scalpel, and the dermis was digested in the tissue solubilizer solution. The applicators, cotton swabs, Kimwipes TM, cell washes, cell ring washes, wash pipets, tape strips, epidermis, dermis, and the receptor fluids were assayed using a Beckman scintillation counter after addition of 15 ml of scintillation cocktail in each vial. RESULTS EFFECT OF pH The percutaneous absorption of lactic acid (pKa = 3.8) from an oil-in-water (o/w) emulsion was measured at aqueous phase pH values of 7.0 and 3.8. The o/w emulsions were applied either as a 2-pl topical film or as a 75-pl "infinite" occluded dose. Evap- orimeter measurements after the application of the topical film on the skin (Figure 1) showed a rapid rise in the water flux followed by a gradual decrease to a steady-state level that was higher than that from an untreated skin. The flux decay curve can be charac- terized by an initial rapid diffusion, which is characteristic of free water followed by a slow diffusion of water that is bound to either the emulsion film and/or the corneum. The TEWL data suggest that the topical 2-pl o/w film hydrated the skin for about 10-15 minutes. In contrast, in the infinite-dose situation, the skin remained hydrated for the duration of the study due to the large reservoir in the donor side and the occlusion provided by the parafilm. The amounts of lactic acid in different tissue compartments after six hours (study duration) are shown in Figure 2 for topical film and in Figure 3 for infinite-dose applications. The results are expressed as the percentage of the applied dose as well as the micrograms of lactic acid per square centimeter (based on a measured skin disc size of 0.64 cm 2) of the skin tissue. Pairwise comparisons were carried out using a two-tailed Student's t-test (17) to obtain the significance of difference in the tissue concentrations at the two pH levels. The effect of pH on the percutaneous absorption of lactic acid depended on the mode of application. When delivered from a topical film, significantly more (p 0.01) lactic acid penetrated the skin at the acidic pH (Figure 2). Decreasing the pH of the 2-pl o/w emulsion film from 7.0 to 3.8 increased the total delivery by four times from 6.5% to more than 25% of the applied dose. The active concentrations in the SC and the epidermis were significantly higher (p 0.01) at the lower pH, and the concentration in the dermis was directionally greater. Similar penetration enhancements at acidic pH
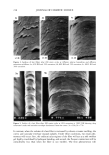
262 JOURNAL OF COSMETIC SCIENCE 6O I untreated skin 2 gl o/w film on skin 5O '•30 • 20 lO 0 5 10 15 Time (min.) Figure 1. Transepidermal water loss measurements (TEWL) after topical application of a 2-pl oil-in-water emulsion film on the skin surface. have been reported for lactic and glycolic acid absorption to viable human skin in i, vitro penetration studies (13). However, the lowering of pH did not have any effect on lactic acid penetration in the infinite-dose situation (Figure 3). In this case, the amount in the SC and epidermis at acidic and neutral pH are quite similar. The difference in the dermal concentrations at the two pH levels was not statistically significant. A similar lack ofpH effect in the infinite-dose situation has been observed for the i, vitro permeation of glycolic (11) and amino (18) acids through mammalian skin. The receptor phase flux at pH 3.8 and 7.0 for finite-dose application is shown in Figure 4. The effect of pH was compared using a completely nested three-way ANOVA (17) (cells within time points within pH level) statistical design. The analysis was done using the SIGMASTAT (Version 2.0) software (Jandel Scientific Software, San Rafael, CA). The analysis indicated that there was no statistically significant difference (p = 0.213). Thus, although there was a directional increase at pH 3.8, the difference in the mean values among the two different levels of pH was great enough to exclude the possibility that the difference was just due to random sampling variability after allowing for the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)