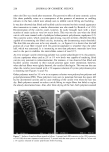
PERCUTANEOUS ABSORPTION OF LACTIC ACID 271 0.06 • w/o/w • o/w 0.25 0.04 0.20 0.15 / 0.00 • - , , • , 0.00 0 I 2 3 4 5 6 Time (hours) Figure 10. Receptor-phase flux profiles and cumulative absorption (pg/cm 2) of lactic acid delivery from a 2-pl finite-dose film of o/w and w/o/w emulsions (n = 7) at pH 3.8. The receptor flux profile for the w/o emulsion was nearly identical to that of the w/o/w. Error bars represent SEM. draw water into the stratum corneum, thus increasing its thickness. For a given lag time, a greater corneum thickness would imply a higher diffusivity. However, more research is needed to resolve this issue. The rate and extent of uptake of lactic acid in various skin strata also depend on product structure. The rank order of the emulsions for tissue delivery of lactic acid from a finite-dose film was o/w w/o/w w/o. This is quite similar to that observed for glucose permeation across a silicone membrane and hairless rat skin in an infinite-dose study (15). The greater efficacy of the o/w emulsion for delivering water-soluble actives might be due to a higher concentration of the active in the external phase. Furthermore, it seems likely that with the o/w emulsion, the corneum was hydrated by the external aqueous phase of the emulsion, whereas with the w/o emulsion, the water was confined within the emulsion drops and hence was not immediately available to the SC. In the case of the w/o/w emulsion, in which the external aqueous phase represents only 20% (w/w), the high internal phase volume fraction rendered the emulsion viscous, leading to decreased water mobility. Although the w/o and w/o/w emulsions delivered less lactic acid to the skin, they might be useful for controlled release of water-soluble actives from topical films (25). The rapid hydration (and consequent water loss from the skin) that occurs when an o/w emulsion
272 JOURNAL OF COSMETIC SCIENCE film is applied to skin often lead to a very high peak ("bolus effect") in the active flux. At low pH, this can cause consumer-perceived negatives such as sting. The lower skin hydration with w/o and w/o/w emulsions should lead to a more controlled skin uptake of lactic acid. The plateauing of the receptor flux for w/o/w and w/o emulsions observed in this study (Figure 10) gives some credence to this hypothesis. ACKNOWLEDGMENTS The authors acknowledge Ms Martha Brown for her generous help in experimental procedures, and thank Unilever Research for permission to publish this work and for providing an industrial fellowship to A. S. REFERENCES (1) A.M. Rosan, The chemistry of alpha-hydroxy acids, Cosmet. DermatoL, 10 (Suppl.), 4-11 (1994). (2) R. Hermitre, Aged skin retinoids and alpha hydroxy acids, Cosmet. Toilerr., 107, 63-67 (1992). (3) L. S. Moy, H. Murad, and R. C. Moy, Glycolic acid peels for the treatment of wrinkles and photoaging, J. Dermatol. Surg. OncoL, 19, 243-246 (1993). (4) E.J. Van Scott and R.J. Yu, Control of keratinization with tx-hydroxy acids and related compounds: I. Topical treatment of ichthyotic disorders, Arch. DermatoL, 110, 586-590 (1974). (5) R. F. Wehr, I. Kantor, E. L. Jones, M. E. McPhee, and L. Krochmal, A controlled comparative efficacy study of 5 % ammonium lactate lotion versus an emollient control lotion in the treatment of moderate xerosis, J. Am. Acad. DermatoL, 25, 849-851 (1991). (6) M.J. Stiller, J. Bartolone, R. Stern, S. Smith, N. Kollias, R. Gilles, and L. Drake, Topical 8% glycolic acid and 8% L-lactic acid creams for the treatment of photodamaged skin, Arch. Dermatol., 132, 631-636 (1996). (7) J. Middleton, Development of a skin cream designed to reduce dry flaky ski n,J. Soc. Cosmet. Chem., 25, 519-534 (1974). (8) R.J. Yu and E. J. Van Scott, Alpha-hydroxy acids: Science and therapeutic use, Cosmet. Dermatol., 10 (Suppl.), 12-20 (1994). (9) W. P. Smith, Comparative effectiveness of tx-hydroxy acids on skin properties, Int. J. Cosmet. Sci., 18, 75-83 (1996). (10) C. M. Ditre, T. D. Griffin, G. F. Murphy, and E.J. Van Scott, Improvement of photodamaged skin with alpha-hydroxy acid (AHA): A clinical, histological and ultra-structural study, Dermatology 2000 Congress, Vienna, May 18-21, 1993, p. 175. (11) M. Goldstein and R. Brucks, Evaluation of glycolic acid permeation through skin, Pharm. Res., 11, S-180 (1994). (12) M. Ohta, C. Ramachandran, and N. D. Weiner, Influence of formulation type on the deposition of glycolic acid and glycerol in hairless mouse skin following topical in vivo application, J. Soc. Cosmet. Chem., 47, 97-107 (1996). (13) M. E. K. Kraeling and R. L. Bronaugh, In vitro percutaneous absorption of alpha hydroxy acids in human skin, J. Soc. Cosmet. Chem., 48, 187-197 (1997). (14) R.L. Bronaugh, "A Flow-Through Diffusion Cell," in In Vitro Percutaneous Absorption: Principles, Fundamentals, and Applications, R. L. Bronaugh and H. I Maibach, Eds. (CRC Press, Boca Raton, FL, 1991), pp. 17-23. (15) L. A.M. Ferreira, M. Seiller, J. L. Grossiord, J.P. Marty, and J. Wepierre, Vehicle influence on in vitro release of glucose: w/o, w/o/w and o/w systems compared, J. Controlled Release, 33, 349-356 (1995). (16) W. C. Griffin, Calculation of HLB values of non-ionic surfactants, J. Soc. Cosmet. Chem., 5,249-256 (1954). (17) P. Armitage and G. Berry, Statistical Methods in Medical Research, 3rd ed. (Blackwell Scientific Pub- lications, Oxford, 1994), pp. 93-153. (18) A. Ruland, U. Rohr, and J. Kreuter, Transdermal delivery of the tetrapeptide hisetal (Melanotropin
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)