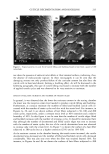
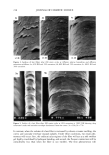
J. Cosmet. Sci., 49, 257-273 (July/August 1998) An in vitro study of the effects of formulation variables and product structure on percutaneous absorption of lactic acid A. SAH,* S. MUKHERJEE, and R. R. WICKETT, Unilever Research US, 45 River Road, Edgewater, NJ 07020 (A. S., S. M.), and University of Cincinnati, College of Pharmacy, 3223 Eden Avenue, Cincinnati OH 45219 (A. S., R. R. W.). Accepted for publication July 15, 1998. Presented in part at the Annual Scientific Seminar of the Society of Cosmetic Chemists, Nashville, May 1-2, 1997. Synopsis The efficacy of lactic acid-containing products is linked to their ability to deliver it to specific skin strata. The penetration of L+ lactic acid to different skin layers of porcine skin from various emulsions was measured in vitro using flow-through diffusion cells. The effects of pH, propylene glycol, product structure, and mode of application on percutaneous absorption of lactic acid were investigated. The absorption of lactic acid from oil-in-water (o/w) emulsions was measured at pH 3.8 and 7.0. The effect of propylene glycol (5%) as a penetration enhancer for lactic acid was also investigated from an o/w emulsion. The emulsion was applied either as a finite-dose 2-pl topical film or as a 75-pl "infinite"-dose occluded patch on a 0.64-cm 2 skin disc. A key finding was that the effects of changes in product compositions such as vehicle pH and propylene glycol on percutaneous absorption of lactic acid depended on the application mode. Increasing the aqueous phase acidity in an oil-in-water emulsion enhanced lactic acid delivery in the finite dose but not in the infinite-dose application. Finite-dose films were significantly more efficient than infinite dose for lactic acid delivery to tissue compartments. The penetration enhancer propylene glycol was more efficacious at the infinite-dose application. However, it also significantly enhanced lactic acid delivery to viable epidermis in the finite-dose application. Finally, the effect of emulsion phase structure on lactic acid uptake was investigated by comparing delivery from oil-in-water (o/w), water-in-oil (w/o), and water-in-oil-in- water (w/o/w) multiple emulsions with identical compositions. The total tissue delivery of lactic acid from the three emulsions was in the order of o/w w/o/w w/o. INTRODUCTION Alpha-hydroxy acids (AHA) such as lactic or glycolic acid are weak organic carboxylic acids in which there is a hydroxyl group at the two or alpha (o0 position along the carbon chain (1). Many AHAs are found in natural products such as fruits and milk that have long been used as ingredients in cosmetic products. For more than thirty years, certain AHAs have been used in cosmetic products as buffering agents at concentrations of 1-2%. More recently, they have been used in cosmetic formulations at concentrations of 2-15% and as superficial chemical peelers by dermatologists at concentrations of 25- * Author for current correspondence. 257
258 JOURNAL OF COSMETIC SCIENCE 70% (2,3). Clinical studies have shown that topical treatment with AHAs, especially lactic and glycolic acids, can moisturize dry skin (4), relieve hyperkeratotic conditions such as moderate xerosis (5), and alleviate signs of photoaging (6). In recent years, this has led to the introduction of large numbers of AHA-containing cosmetic products in the marketplace with great consumer acceptance. The efficacy of AHA-containing products is linked to their ability to deliver these actives to specific skin strata. Depending on the type and formulation, the AHAs can act either at the stratum corneum or at the deeper, viable tissue level. AHAs enhance the extensibility and the water-binding capability of stratum corneum (7). They may modu- late stratum corneum (SC) formation through diminished cellular cohesion between corneocytes at the lowest levels of the SC (4,8). The benefits of smaller water-soluble AHA homologues such as (L+) lactic or glycolic acid is believed to be related to their ability to enhance epidermal cell turnover (9) and collagen and mucopolysaccharide synthesis in the dermis (10). It is well known that percutaneous absorption depends not only on the nature of the active but also on the vehicle composition. AHAs are currently formulated in a wide variety of cosmetic creams and lotions with differing pH, compositions, or product structure. Although all these products claim efficacy, very little is known about skin absorption of AHAs from complex emulsion systems. Only a few reports (11-13) of systematic study on uptake of AHAs to various skin strata from topical application have been published. Moreover, results of some of these studies appear to be in conflict. For example, in one study (11), decreasing the pH of the aqueous delivery vehicle from 7.4 to 3.8 did not affect skin penetration of glycolic acid (pKa = 3.8), whereas in another (13), changing the pH of an oil-in water emulsion vehicle from 7.0 to 3.0 led to a significantly greater glycolic acid delivery. The objective of this study was to gain insights into how absorption of small water- soluble AHAs into various skin strata could be modulated by compositional and struc- tural changes in the delivery vehicle. Percutaneous absorption of (L+) lactic acid (pKa = 3.8) through dermatomed porcine skin was measured in an in vitro flow-through Bro- naugh diffusion cell (14) using well characterized emulsions as test vehicles. The effects of vehicle pH and propylene glycol (as a penetration enhancer) on skin permeation of lactic acid were studied using an oil-in-water (o/w) emulsion. The o/w emulsion was applied either as a 2-pl topical film or as a 75-pl "infinite"-dose (i.e., in large excess) occluded patch on a 0.64-cm 2 skin disc. Comparison of the results provided insights into penetration pathways of lactic acid in stratum corneum. The effect of vehicle structure on delivery was studied by comparing distributions of lactic acid to different skin strata from topical application of oil-in-water (o/w), water- in-oil (w/o), and multiple (water-in-oil-in water [w/o/w]) emulsions. The composition of the emulsions was kept constant to minimize the effect of formulation variation on AHA delivery. The role of emulsion structure on dermal delivery of a water-soluble active, glucose, in an infinite-dose situation has been studied (15). No similar study for AHAs, either in an infinite-dose or in consumer-relevant finite-dose application, has been reported in the literature.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)