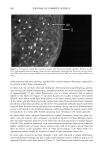
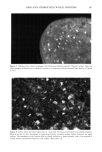
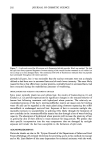
162 JOURNAL OF COSMETIC SCIENCE product A for the instrumental measurements. Sites treated with product A were not significantly different from the untreated control sites. Experiment 2---FCAT with and without hydration. The objective of this experiment was to determine the effect of hydration on the ability of the FCAT to detect product mildness differences. In this study, panelists underwent a four-day pretreatment period during which patches were applied to test sites designated for semi-occlusion. Patches consisted of the modified diaper patch with 60 ml distilled water. The washing cycles were conducted on days 1 through 5 in a manner identical to those in Experiment 1. Patches were applied nightly after the final wash cycle of each day on days 1 through 4. Panelists were instructed to remove the patches at least one hour prior to arriving for the first washing cycle of each day. Instrumental and visual scoring was conducted at the same time points as in the previous experiment. Additional scoring was conducted prior to the pretreatment period. Visual scoring for erythema and dryness were done using seven- point scales (0-6), as described in the Materials and Methods section. The washing procedure was terminated at any test site with a visual score for either erythema or dryness of 3. If an early termination was necessary, instrumental measurements were conducted at that site at the time of termination. Results are shown in Table III. All effects were increased when skin was hydrated through semi-occlusion except water loss for test product A. As in the first experiment, Products A and C were significantly different in all four parameters on the normal (non-occluded) test sites. On the hydrated sites, only water loss was significantly dif- ferent. However, Product C was directionally worse (i.e., less mild) than A for the remaining three parameters. Experiment 3--Extended FCAT with and without hydration. The objective of this experi- ment was to extend the duration of the study in an attempt to achieve statistically significant differences in all parameters. There was no conditioning or pretreatment period in this experiment. In order to better reflect actual consumer habits and practices for use of baby wipe products, the washing cycles were reduced to 15 seconds each, except for the final washing cycle on each day, which was 40 seconds. Washing cycles were conducted four times daily for 13 consecutive days, and twice on day 14. Hydration was achieved with the J&J bandage system and 10 ml distilled water. The patch was applied nightly, and removed one hour prior to the first wash of the next day. Instrumental measurements were conducted prior to the first washing session on day 1, after the second washing session on day 5, and after the final washing session on day 14. Visual scoring for erythema and dryness was conducted prior to the first and third washing sessions on days 1-13, and prior to both the first and second washing sessions on day 14. Seven-point scales (0-6) were used for erythema and dryness. The washing procedure was terminated at any test site with a visual score of •5. If an early termi- nation was necessary, instrumental measurements were conducted at that site at the time of termination. Results are shown in Table IV. All effects were increased when skin was hydrated through semi-occlusion. The two products were significantly different in all four pa- rameters on both normal (non-occluded) and hydrated (semi-occluded) test sites, with Product C being less mild than Product B. Substantial differences in intermediate scores were observed throughout this study. An example of the intermediate erythema scores is shown in Figure 1. The intermediate
MODIFIED FCAT 163 scores for dryness showed a similar pattern. One goal of this program was to develop a practical, cost-effective test method for measuring mildness differences on hydrated skin. Therefore, statistical analyses were conducted on the intermediate scores to determine if an experiment of shorter duration would provide statistically significant results for all endpoints. Recorded scores at all time points starting with day 5 (scoring time point 10) in the 14-day experiment were analyzed. A summary of the days that yielded statistically significant results is shown in Table V. As shown in this table, the study consistently yielded statistically significant results for all endpoints except dryness on both normal and hydrated skin at all time points starting at day 5. After nine days, the dryness endpoint was also significantly different. The instrumental measurements were con- ducted on days 5 and 14, and were significantly different on both days. DISCUSSION A Modified FCAT has been developed that is capable of producing statistically signifi- cant differences between different baby wipe products in erythema, dryness, redness, and TEWL on both hydrated and normal skin. A patch system was developed for hydrating the skin that was convenient, cost-effective, and well accepted by the panelists. The first three experiments in the patch system development phase demonstrated that distilled water was roughly equal in effectiveness to synthetic urine in producing a hydrated environment, and a patch consisting of the J&J bandage with gauze and Blenderm tape provided adequate hydration when compared to the other patch systems tested, includ- ing the modified diaper patch. Panelist acceptance was an important consideration for the patch system. Comments were solicited from the panelists with regard to the comfort and wearability of the various patch systems. The modified diaper patch was not well received. It was consid- ered too large and unsightly for a test requiring several days. The 60 ml of fluid required to saturate the patch made the patch too heavy to be worn comfortably. Bathing with the modified diaper patches resulted in patches soaked with water. Not only did this contribute to panelist discomfort, it resulted in spuriously high TEWL readings, neces- sitating exclusion of some subjects from the final analysis. In addition, the modified diaper patches had to be hand cut from commercial diapers, which added considerably to the overall study costs. The patches using the J&J bandage were well received by the panelists however, without reinforcing tape these did not stay in place overnight for some, resulting in several panelists being dropped from these study groups. The gauze was a necessary addition in order to achieve a skin environment similar to that achieved with the modified diaper patches. In the FCAT modification phase, the study summarized in Table II confirms that the FCAT has enough sensitivity to detect differences in the skin effects of products on normal (non-hydrated) skin in an experiment of only five days' duration. However, we expected that differences in mildness would be more challenging to detect on hydrated skin due to the background level of irritation. This increased level of irritation is demonstrated in Tables III and IV. In every instance but one (TEWL scores for Product
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)