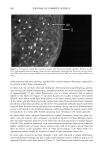
164 JOURNAL OF COSMETIC SCIENCE 3.00 -- 2.50 2.00 1.50 1.00 0.50 0.00 .... Product ._ ./ø . 3.00. I 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 Scoring Time Point 2.50. ---- Product 2.00 ........... • 1.50 1.00 /'/ 0.50 0.00 I 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 b Scoring Time Points Figure 1. 14-Day Modified FCAT: Visual Scores for Erythema for Products B and C. In this test of 14 days' duration, products were compared on both normal (a) and hydrated (b) skin. Visual scoring was conducted twice daily (i.e., prior to the first and third washing cycle of each day). Therefore, scoring time points 1 and 2 occurred on day 1, scoring time points 3 and 4 occurred on day 2, etc. Data for the final scores for all four parameters (erythema, dryness, redness, and TEWL) are given in Table IV.
MODIFIED FCAT 165 B in Table IV), hydrated skin showed higher scores for all four endpoints when treated in the same manner as normal skin. The experiment summarized in Table III confirms that differences in skin effects are more difficult to detect on hydrated skin. In this experiment five days' duration, we were once again able to detect significant differences in all four endpoints on the normal skin sites. However, on the hydrated skin sites, only the TEWL measurements were signifi- cantly different. The other three endpoints were directionally different, but did not reach significance. By increasing the overall duration of the test to 14 days, as in the experiment summa- rized in Table IV, we were able to achieve significant differences in all four endpoints on the hydrated skin sites. These differences were detected in spite of the fact that the duration of each product application was reduced to better reflect the actual consumer habits and practices of product use. Eliminating the pretreatment period did not appear to compromise the ability to hy- drate the skin sites, as demonstrated by comparing the normal to hydrated value for each product in the 14-day study presented in Table IV, where no pretreatment was used. For all four endpoints, the hydrated skin showed an increase in irritation. A final step in development of this method was to determine the optimum test duration and number of panelists required to achieve significant results. It was possible that a test of 14 days using 80 subjects, such as the one presented in Table IV, was more than absolutely necessary. Statistical analyses of the intermediate points from the experiment presented in Table V indicated that the duration of the test could be reduced to nine days and still produce significant differences between these two products for all endpoints. Subsequent studies have indicated that a test of seven days' duration involving approximately 60 subjects provides consistently reliable results. Table V Summary of Statistical Differences Between Products B and C At Different Time Points in the 14-Day Modified FCAT Day 5 Day 7 Day 8 Day 9 Day 14 Normal skin Erythema Yes • Yes Yes Yes Yes Dryness No Yes Yes Yes Yes Redness Yes -- -- Yes TEWL Yes -- -- -- Yes Hydrated skin Erythema Yes Yes Yes Yes Yes Dryness Yes No No Yes Yes Redness Yes -- -- -- Yes TEWL Yes -- -- -- Yes This is a summary of significant differences at the interim scoring time points for the 14-day study presented in Table IV. Statistical significance for erythema, dryness, and TEWL readings was determined using a Wilcoxon's signed rank test. Statistical significance for chromameter readings was determined using a two-sided paired t-test. • "Yes" indicates a significant difference between Products B and C (p 0.05). "No" indicates no significant difference.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)