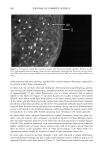
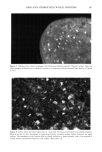
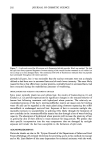
OXYRADICALS FROM PHOTOIRRADIATED HAIR 179 10 Gauss Figure 7. Effect of cinnamic acid on the DMPO adduct from irradiated red hair: 0.02 g red hair in 1 ml of 20 mM DMPO solution. The spectrometer settings are the same as in Figure 6. a. No irradiation. b. Irradiated for 10 min. c. 10 mM cinnamic acid added, irradiated for 10 min. Propagation: RS' + 02 • RSOO' RSOO' + RSH • RSOOH + RS- RS' + RSH • RSSR'- + H + RSSR'- + 02 • RSSR + 02'- 02 + RSH • H202 + RS'- Reduction of hydrogen peroxide: RSSR-- + H202 • RSSR + OH- + OH Termination: 2RS' --- RSSR RS' + RSSR'- • RSSR + RS- 202'- + 2H + • H202 + 02 Scheme 2
180 JOURNAL OF COSMETIC SCIENCE cystine residues also may cause the hair to lose some of its natural ability to absorb UV light. Exposure to light induces the oxidative destruction of disulfide and phenolic side chains of the polypeptide chains. The light quanta induce the formation of free radicals that attack the disulfide bonds by chain reactions. Lengthy exposure to sunlight, especially in the summer or in southern regions where the exposure to sunlight is stronger and longer, can bring 20-40% weakening of the hair structure. Air- and water-borne pol- lution can also contribute to the oxidative damage of hair. In urban areas a great deal of polynuclear hydrocarbons and heavy metal oxide-containing pollutants are airborne and adsorb onto the hair surface. Many of these materials are light sensitizers capable of converting oxygen molecules into oxygen species. These attack and oxidize hair and degrade it (2). A particularly interesting aspect of the present study is the effect of cinnamic acid. Cinnamates are an important class of sunscreen, perhaps most notably as octylmethoxy- cinnamate. Since our studies are carried out in aqueous media where octylmethoxycin- namate would not be soluble, we used cinnamic acid as a functional model for cinnamate sunscreens. The absence of a DMPa-aH signal for hair irradiated in the presence of buffered 10 mM cinnamic acid (Figure 7) is an indication that the additive either prevents the formation of oxyradicals (0' 2- or OH') or competes effectively for them with the spin trap. Since, at the concentrations and wavelengths used here, cinnamic acid has no significant absorbance, the mode of action in quenching the oxyradical signal is not simply by blocking or absorbing light. An alternative mode of action for cinnamic acid is scavenging Ha,. Other aromatic hydrocarbons such as benzoic acid are used as Ha- scavengers due to their ability to compete for Ha. via aromatic hydroxylation. This observation may have significant implications for the use and development of sunscreens. Sunscreens used in skin care are not always desirable in hair care since they tend to be lipophilic and, as such, impart an undesirable oiliness to the hair. Furthermore, strat- egies in developing sunscreens for skin care may not yield materials optimal for hair care since different mechanisms may be operative and/or have different levels of importance in one versus the other. For example, the reaction of skin to UV-Vis radiation involves immunological responses not possible for hair. CONCLUSION Reduced oxygen radicals have been postulated during photoirradiation of hair, and the results of our studies provide direct evidence for the production of oxyradicals from human hair under UVA-visible irradiation. In addition, we have shown the value of combining direct ESR measurements and ESR spin trapping with a novel fluorescent probe for hydroxyl radical production. Terephthalate ion proves to be a convenient probe for the study of the production of hydroxyl radicals from irradiated samples of melanin and human hair, with potential utility in other areas. While we must emphasize the need for carefully prepared blank experiments, the relatively high excitation wavelength for HTA allows the presence of a variety of additives, including the radical scavengers used in this study and in our experiments with melanin (25). Our results indicate that bleached and red hair give a greater net yield of hydroxyl radical than brown hair under irradiation, reflecting the role of eumelanin in darker hair
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)