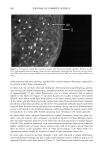
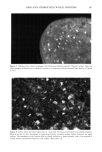
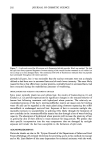
HAIR AND HYDROLYZED WHEAT PROTEINS 203 REFERENCES (1) M. Gamez-Garcia, Effects of some oils, emulsions, and other aqueous systems on the mechanical properties of hair at small deformations, J. Soc. Cosmet. Chem., 44, 69-87 (1993). (2) S. P. Chahal, N. I. Challoner, and R. T. Jones, Moisture regulation of hair by cosmetic proteins as demonstrated by dynamic vapour sorption: A novel efficacy testing technique, Proc. 14th Latin- American and Iberian Cong. Cosmet. Chem., Santiago Chile, 45-56 (1999). (3) R. T. Jones and S. P. Chahal, The use of radiolabelling techniques to measure substantivity to, and penetration into hair of protein hydrolysates, Internat. J. Cosmet. Sci., 19, 215-226 (1997). (4) T. Wilson, Ed., Con•bcal Microscopy (Academic Press, San Diego, 1990). (5) J. A. Swift, "The Detection of Pores and Holes in Hair by Electron Microscopy," in Hair Research fir the Next Millennium, D. J.J. Van Neste and V. A. Randall, Eds. (Elsevier Science BV, Amsterdam, 1996), pp. 109-112. (6) J. A. Swift and B. Bews, The chemistry of human hair cuticle. Part 3. The isolation and amino acid analysis of various subfractions of the cuticle obtained by pronase and trypsin digestion,J. Soc. Cosmet. Chem., 27, 289-300 (1976). (7) J. A. Swift, "The Histology of Keratin Fibers," in The Chemistry of Natural Protein Fibres, R. S. Asquith, Ed. (Plenum Press, New York, 1977), pp. 81-146. (8) P. Kassenbeck, Das haar und seine struktur, Wella AG, Darmstadt, Germany (1984). (9) J. A. Swift and A. K. Allen, "Swelling of Human Hair by Water," in Abstracts of Sth Internat. Hair Sci. Symp., Kiel, Germany (Deutsches Wollforshungsinstitut, Aachen, Germany, 1992). (10) M.A. Pykett and J. A. Swift, Schutz von Haarbruch nachweisen: Ein neues Testverfahren fiir eine bessere Datenbasis, Parr. u. Kosmetik, 79, 38-40 (1998).
j. Cosmet, sci., 51,205-207 (May/June 2000) Abstracts Journal of the Society of Cosmetic Chemists Japan (as published in Vol. 33, No. 2, 1999) The Mechanism of Desquamation in the Stra- tum Comeurn and Its Relevance to Skin Care Junichi Koyama**, Jotaro Nakanishi**, Junko Sato**, Junko Nomura***, Yumiko Suzuki**, Yoshiko Masuda**, Yasuhisa Nakayama** Life Science Research Center**, Basic Research Cen- ter***, Shiseido Co., Ltd. For healthy people, the most common skin problem is the occurrence of visible scales on the skin surface. This phenomenon is commonly seen on dry skin. Many morphological and biochemical studies on the stratum comeurn have revealed the aspects of skin. However, we still do not know why and how scales appear on the skin surface, except that a defect of the desquamation process in the stratum corneum must be involved. In this study, we examined the mecha- nism of desquamation, to establish what factors in- fluence the mechanism and what treatments might be effective for skin care. We found two types of proteases, trypsin type (30 kDa) and chymotrypsin type (25 kDa), in stratum corneum (SC). cDNA cloning followed by nucleotide sequence analysis re- vealed that the chymotrypsin-like protease corre- sponded to the reported chymotrypsin-like enzyme in stratum corneum (SCCE). Trypsin-like protease corresponded to trypsinogen IV and we found new type of trypsinogen. Desmosomes in SC sheets were digested and SC sheets were dissociated into indi- vidual intact cells in buffer solution, whereas heat- treatment or addition of inhibitors of these proteases to the buffer solution prevented the degradation of desmosomes and the cell dissociation. Leupeptin or chymostatin retarded the cell dissociation only about half as effectively as aprotinin, but a mixture of the two inhibited stratum corneum sheet degra- dation as potently as aprotinin. These results sup- port the hypothesis that desmosomes play a key role in the adhesion of SC cells, and the digestion of desmosomes by these two types of serine proteases leads to SC desquamation. An age-related decrease in the activity of the trypsin-type protease was ob- served in normal subjects. Digestion of desmosomes in SC by the proteases was influenced by the water content in SC. Lower humidity (lower water content in stratum corneum) resulted in a decrease of des- mosomal degradation. Our studies demonstrated that desquamation was influenced by two factors. One is water content in the stratum corneum. Under the low water condition enzymes cannot work well, even if the contents of the enzymes are normal. In this case, humectant treatment was effective by sup- plying water to the stratum corneum. The other factor is a decline in the activity of the proteases themselves. This can be seen in diseased or aged skin. Humectant treatment is not sufficient in this case compounds that accelerate desmosomal diges- tion independently of the water content in the stra- tum corneum are required. Derivatives of dicarbox- ylic acid are thought to be the candidate for such ingredients. Key words: stratum corneum, desquamation, prote- ase, dry skin, desmosome, o•-hydroxy acid Evaluation of Pigmentation by Multispectral Image Analysis (Second Report): Reconstruc- tion of Pigmented Images Based Only on the Melanin Component in the Skin Yukiko Kawaguchi, Osamu Kaneko, Institute of Beauty Sciences, Shiseido Co., Ltd. Conventional digitized images of pigmented skin include both coloring caused by melanin and color- ing contributed by other skin factors. To accurately quantify the darkness of pigmented skin, only mela- nin should be considered. Coefficients for weighting M 1 and M 2 for the reflected light constituent factors 205
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)