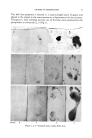
USE IN COSMETICS OF FURYLGLYCINE 79 isolated forms, applied locally or otherwise, do activate the cutaneous "nourishing" process, favoring granulation and healing. EXPERIMENTAL Chemical Initially we have tried to synthesize furylglycine according to the classical Streker synthesis. The reaction takes place in two fundamental phases: by the action of ammonia, ammonium chloride, potassium cyanide with a ketone or an aidehyde (in this case furrural), one can obtain the formation of the cor- responding amino nitrile which by successive hydrolysis forms the cor- responding amino acid. However, from the very beginning of the first phase, instead of the amino nitrile we have obtained some black resins. Therefore, we had to adopt various modifications, such as working with a 50 per cent hydro-alcoholic solution trying different emulsifiers in nitrogen atmosphere and finally at low temperature, but always with unsuccessful results. We have now adopted the method of synthesis suggested by Harvill and Herbst (4) and by Henze and Speer (5) which consists initially in the synthesis of Biickerer for the preparation of the hydantoins from aldehydes or ketones and successive opening and degradation of the nucleus with formation of the amino acid. We have reacted 0.02 moles of furfural with 0.08 moles of ammonium carbonate and 0.04 moles of potassium cyanide in 50 cc. of 50 per cent alcoholic solution. The mixture is heated for about two hours at 58 ø to 60øC., and then the solution is concentrated to two-thirds of the initial volume. After the acidification with hydrochloric acid, the 5-furylhydantoin is crystallized in an ice-bath. The product has a melting point of 147øC., and is obtained in a yield of 65 per cent. The furylglycine is obtained by hydrolysis of 5-furylhydantoin and by means of concentrated solution of barium hydroxide. The melting point is 212 ø to 213øC., with decomposition. The yield is 52 per cent. The reaction scheme is the following: KCN Ba(OH)2 O O COOH Cosmetic. The two referred chemicals have been tested in a facial mask on one-half of the face with the other half used as the control. Then general cosmetic formulas were made as follows: the 5-furyl- hydantoin, which is partially liposoluble, has been previously dissolved in oleyl alcohol the furylglycine, definitely hydrosoluble, is dissolved
80 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS in the watery phase. Both were gelatinized with bentonite 300 mesh with 10 per cent of sorbitol acting as the hurriectant. After several tests made at the Dermatologic and Esthetic School of Milan and in other beauty laboratories, the following results were obtained: (a) The best concen'trations were found to be 1 per cent of 5-furyl- hydantoin and of 3 per cent furylglycine. (b) The skin effects have been very evident for both chemicals. While the 5-furylhydantoin has a hydrating and tonifying action on the skin, the furylglycine has a clearing and smoothening effect on the skin. Both chemicals have also a remarkable "nourishing" effect on tissues. The tests through magnification revealed that the thin fissures of old and tired skins appear much less evident, while skin smoothness is increased. (c) In cream, "nourishing" treatments and other cosmetics containing additional skin active constituents, the skin health improvement results are even more evident. (d) Neither incompatibility nor allergy have been noticed up to now from the use of these two chemicals. (e) The tests on the toxicity of the two chemicals have given results similar to those of the normal amino acids. Consequently, their use appears harmless. SUMMARY In accordance with the tests carried out by us, $-furylhydantoin and furylglycine have proved to be et•caciously active products for the skin. Furylhydantoin (liposoluble) is different in its action from furylglycine because of its major hydrating power, while the amino acid has a marked action in minimizing skin lines. Both products have a remarkable "nourishing" and "regenerating" action. In view of the low cost convenience of these products, we feel certain that they can find wide application in cosmetics. REFERENCES (1) Ciocca, B., Rovesti, P., and Roccheggiani, G., "Azi0ne cutanea di a[cu•i •uovi ami•o-acidi a[icidici di $i•te$i." Presented at the XII International Congress ooe Esthetics and Cos- metology held in Venice, May, 1958. To be published. (2) Fischer, Albert, "Die Bedeutung d. Aminosauren F. die Gewebezellen in vitro," Acta Phys. Scand., 143 (1941). (3) Dunlop, A. P., and Peter, F. N., "The Furans," Monograph Series 119, New York, The Reinhold Publishing Co. (1953), p. 598. (4) Harvill, E. K., and Herbst, R. M., •. Org. Chern. 9, 21 (1944). (5) Henze, H. R., and Speer, R..[., •. Am. Chem. $oc., 64, 522 (1942). (6) Hermann, G., Kosrnetik-Parfurn-Drogen Rundschau, 9/10 (1956). (7) Hermann, G., Ibid., 3/4 (1957).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)