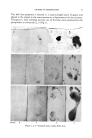
HUMAN SKIN SURFACE LIPIDS 89 three pertinent assays--weight, acid number (A.N.) and spreading index (S.I.)--every sample was processed as follows. After the total volume (10 ml.) of fat free ethyl ether used to collect the material in our cylindrical receptacle had in a 10 ml. measuring flask slowly evaporated at 40øC., the residue was redissolved in 10 ml. of fat free petroleum ether. Under precautions necessary to avoid transfer of scales, etc. (6, 11), 5 ml. of this volume were used for gravimetric deter- mination of the lipid quantity,*t 3ml. for assaying the acid number and the remainder for assaying the spreading index. The A. N. was assayed in a manner similar to the procedure of Hodgson- Jones and Wheatley (12), who in turn rather closely followed the method described by Moyle, Baldwin and Scarisbruck (13). However, because of the much smaller lipid quantity obtainable from the different sites to be investigated separately, several modifications had to be introduced. Thus, for the titration it was found necessary to reduce the concentration of KOH in methanol from 0.005 N to 0.002 N.• Smaller volumes, more- over, of solvent (chloroform) and of indicator had to be employed. Briefly, we proceed in the following manner: The 3 ml. aliquot of the solution in petroleum ether is placed in a wide- necked 30 ml. Erlenmeyer flask and evaporated again at 40øC. After cooling, 2 ml. of chloroform and one drop of 0.04 per cent cresol red in methanol are added to the residue. The solution is carefully shaken. In order to remove the COs present a stream of gaseous nitrogen is carefully bubbled through the chloroform solution for thirty seconds prior to titra- tion. This is continued also through the period of titrating it must be done slowly to avoid volatilization. The titration out of a microburette (one drop equals 0.01 ml. of the KOH solution in methanol) likewise is carried out slowly, since neutralization and color change, as a rule, do not occur immediately. The turn from greenish-yellow to a reddish-yellow is the end point. The addition of one drop in excess produces a distinctly purple color. Under our laboratory conditions it is necessary to titrate about four "blanks" of chloroform, 2 ml. each, immediately prior to any assaying of samples. There are day to day variations of the blank titres from 0.02 to 0.03 or even 0.04 ml. of KOH, which must be deducted from the volumes required for neutralizing the solution under test. From time to time it has been useful to perform control titrations of' different known concentrations of stearic acid in chloroform. * TCY (Key Board) Balance, William Ainsworth & Sons, Denver, Colo., sensitivity 0.01 mg. t Weighing of the residue was performed in 6 ml. aluminum foil cups, 3 cm. in. diame- ter, "Crinkle Cups," Muller Paper Goods Co., Inc., Long Island City, N. ¾. it Prepared by courtesy of Joseph R. La Vieres, La-Mar Laboratories, Inc., New York, N. ¾.
90 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS z• o • ¸
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)