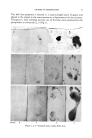
120 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS These preparations were coded and panel tested among a group of women office workers. The panel members were asked to shampoo their hair in their normal manner once weekly but to refrain from using any hair dress- ing, wave set or rinses. Each shampoo was used over a two-week period and the panel members were then checked for their opinion as to latherability, manageability, luster, cleanliness and ease of wet comb- ing. The panel members all lived in a hard water area (approximately 150-300 p.p.m. hardness) and used unsoftened water in this test. The results obtained are presented in Table 2. For comparison, a solution of triethanolamine coconut soap at a pH of 8.5, prepared from a stripped coconut fatty acid and containing approximately 12 per cent active anion, was also included in this test. From the data in Table 2 it is interesting to note that soap alone was the least acceptable from the standpoint of hair luster, manageability and cleanliness although quite acceptable as to latherability. One'would expect that there should be a direct correlation between hair cleanliness and luster but this apparently is not the case. For example, sodium "coco" methyl taurate was rated best for "cleanliness" yet is only intermediate in luster. Perhaps cleanliness is a combination of factors related to degree of "degreasing" as well as the amount of insoluble calcium TABLE 3--A COMVARXSON or LABORATORY CLEANSING DATA FOR SELECTED DETERGENTS WITH PANEL TEST RESULTS Detergent Grease Panel Test Removal, % (% Voting Laboratory Good Cleansing Tests* Action) Triethanolamine "lauryl" sulfate 92 Protein fatty acid condensate 77 Triethanolamine dodecyl benzene sulfonate 73.7 Sodium octyl phenoxy polyoxyethylene sulfonate 81.7 Ammonium lauroyl isethionate 92.8 Sodium "coco" methyl taurate 95.0 Coconut soap 90.3 91 74 73 80 91 100 66 * Laboratory test data obtained from Barnett-Powers work (2, 4, 6), using 0.25% active detergent in tap water at 38øC. and magnesium salts deposited on the hair. In Table 3, there is presented a comparison of laboratory cleansing action and panel members' opinions on a few of the detergents tested. Surprisingly good correlation was ob- tained between laboratory cleansing tests and use tests for most of the detergents tested except soap. In the case of soap the heavy, dulling, lime soap deposit on the hair, undoubtedly, influences opinion as to cleanliness. It should be noted that the Barnett-Powers tests cited here were conducted in New York City tap water which is very soft indeed and much softer than the 150-300 p.p.m. tap water used in these panel tests. The solubility characteristics of the detergents studied in 350 p.p.m.
ROI.E OF DETERGENTS IN SHAMPOOS 121 hard water are shown in Table 4. These data indicate that, while many detergents show good solubility in hard water at higher concentrations, they precipitate out on dilution or during rinsing. Undoubtedly, the heavy metal salts of many of these detergents are insoluble per se in water but are solubilized in an excess of the soluble detergent salt. Where detergents precipitate in hard water they will be potential hair dulling agents. TABLE 4--SOLUBILITY CHARACTERISTICS OF SELECTED DETERGENTS IN 350 P.P.M. HARD WATER* DeterRent Solubility as Judged by Clarityj' of Solution at 0.5% level:J: 0.1% level+ + Triethanolamine "lauryl" sulfate Triethanolamine lauryl beta amino propionate Triethanolamine dodecyl benzene sulfonate Protein fatty acid condensate Sodium octyl phenoxy polyethoxy sulfonate Sodium lauroyl sarcosinate Sodium lauryl polyethoxy sulfate Sodium caproyl imidazoline carboxylate Ammonium lauroyl isethionate Sodium "coco" methyl taurate Triethanolamine coconut soap Clear Very cloudy Clear Clear Slightly cloudy Cloudy Clear Clear Slightly cloudyõ Cloudyõ Clear Very cloudy Clear Clear Cloudy Cloudy Cloudy Cloudy Clear Clear Very cloudy Very cloudy * Prepared by dissolving 0.259 grams of calcium chloride (anhydrous) and 0.281 grams of magnesium chloride (hexahydrate) in 1 liter of distilled water. j' Clarity was judged five minutes after solution was prepared. :]: Percentage refers to anion. õ This deterRent is slightly cloudy at these concentrations in distilled water. A comparison between panel opinions as to latherability and laboratory test data, using the Barnett-Powers lathPromPter, is presented in Table 5. The correlation is not too good when one considers the laboratory data obtained with soft or hard water. However, it is very enlightening to see what a close correlation is obtained when synthetic sebum is used as part of the laboratory test. TABLE 5--A COMPARISON OF LABORATORY LATHERABILITY DATA* fOR SELECTED DETERGENTS WITH PANEL TEST RESULTS DeterRent .... Laboratory Latherability--- ß Panel Test (ml. of Lather) Results In In 175 In Soft Water (% Voting Soft p.p.m. Hard Containing 2% Good Water Water Synthetic Sebum Lather) Atkyl sulfate 86 94 203 100 Triethanolamine dodecyl benzene sul- fonate 52 70 150 86 Sodium octyl phenoxy polyoxyethyl- ene sulfonate 57 55 92 50 Protein fatty acid condensate 73 130 100 79 Sodium caproyl imidazoline carboxyl~ ate 47 57 120 81 Soap 49 44 399 92 * These data, obtained from reference No. 2 Tables 1 and 4, are based on 3% solutions of deterRent.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)