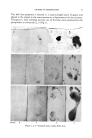
DESIGN OF COSMETIC SUN SCREENS 101 It is important to note that absorption can take place only if the photon corresponds to the difference in energy between two possible energy levels of the molecule. The change from one such permissible level to the other is termed an electronic transition. Each transition gives rise to an absorption line of very narrow, though finite, width and for each such electronic transition, there is a progression of closely spaced absorption lines corresponding to different possible combinations of vibrational and rotational levels belonging to the same two electronic levels. In complex molecules, the number of possible combina- tions of vibrational and rotational levels are so great that the linear fine structure is blurred. In solutions, and in the condensed states, vibrations and rotations are blurred still further by solute-solvent interactions and by molecular association of the close-packed molecules. This gives rise to a diffuse absorption band which is observed in place of the system of discrete absorption lines. FIG.3, INTENSITY OF MDSUIVlV1ER NO(DN•Y SUN- 5 SKYI_IGHT AT • LEVEL ½' ---WASHINGTON D.C. ,,z•'.' -•-TUCSON ARIZ. •,- - ..--CLEVELAND O. IOOC 300 320 340 36•) 3@0 400 WAVELENGTH - MILLI MICRON S WAVE LI-NGTH-M[J 280 290 297,5 •'• I I I I _ •' • FIG.4. ERYTHEMAL I •-- EFFECTIVENESS OF - - ULTRAVIOLET RADIATION _ RELATIVE TO 2970 A.U, I -- , 330 320 340 350 380 400 WAVELENGTH - MILLIMICRONS ENERFoY- K,F.,AL/6M- MOLE 103,2 99,4 96.9 315 91.4 320 90.4
102 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Braude (1) points out that the absorption maximum for a given molecule corresponds to the most probable transition, and the value of X,•x. is one of the most characteristic parts of the absorption curve. For a cosmetic sun screen, the location of the absorption maximum should therefore correspond to those wavelengths of sunlight which have a major erythemal effect on human skin. Solar radiation is known to extend in a continuous band throughout the entire electromagnetic spectrum. Were it not for the absorption of the lower wavelengths by our atmosphere, which absorbs virtually all radiation of wavelengths shorter than 290 millimicrons (mu), it is doubtful that life, at least as we know it, would have been possible on Earth. At any rate, the major erythemal wave- lengths penetrating the atmosphere to sea level extend from about 290 to 315 mu. From equation 1 it is calculable that these wavelengths correspond to transition energies of 91.4 to 99.4 kcal./gm.-mole. At the wavelength of maximum erythemal effectiveness, 297.5 m/•, the corre- sponding transition energy is 96.9 kcal./gm.-mole. A transition with zXE of 90.4 kcal./gm.-mole will have an absorption maximum at 320 mu, a region where absorption is undesirable because it impedes tanning. At the other end of the erythemal range, a transition energy of 103.2 kcal./gm.-mole results in an absorption maximum at 280 mu, too low to be cosmetically useful. It becomes apparent from these figures that the allowable transitions that will result in a useful cosmetic sun screening fall within an exceedingly narrow range of energy requirements. It is not surprising then that few molecular species have been found which will serve satisfactorily as screens for human use. The extent of the absorption of any particular wavelength depends further on the molecular geometry of the molecules. The quantitative measure of completeness of absorption is given by the molecular extinction coefficient, e,•x., which is defined by the equation: • .... = (O.D./cl)= (100f%T)/cl) (5) where O.D. = optical density -- log (100/%T) %T = per cent transmission c = concentration of the screen, gm.-moles/liter, and l = thickness of the absorbing layer, in centimeters. The value of ema, x ' iS as characteristic of a molecular species as is the value of The absorption coefficient, e .... , is determined by the transition prob- ability, in the statistical sense. That is: when a photon of radiant energy strikes a molecule, what is the statistical probability of absorption of the photon? This transition probability is given approximately by Braude (1) as
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)