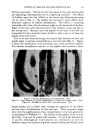
EXPERIENCES IN SAFETY TESTING OF COSMETICS 171 On the basis of this single experience, it is not possible to make any generalizations concerning the safety of a given raw material or even a combination of raw materials. It can be stated, however, that it should not necessarily be concluded* that the use of "safe" ingredients will in fact produce a safe product. The knowledge gained during the research and development with the above mentioned make-up product was successfully used to pinpoint the irritating agents in another totally unrelated product. The same grade of mineral oil was used in combination with a small amount of a nonionic wetting agent in another cosmetic and produced a significant number of primary irritation reactions in a closed patch test. In view of past ex- perience, reformulation was a simple matter of switching to a heavier grade oil. A subsequent patch test which yielded no reaction reflected the increased safety of the product. CONCLUSIONS The authors have illustrated attempts to arrive at a rational approach to cosmetic safety testing. It has been shown that use of animal tests is of limited value, except in the case of extremely irritating preparations. Further, for animal tests to be of any value at all, they should be followed by tests on human volunteers since complete reliance on the animal tests may be misleading. It is of equal importance that safety tests on humans be carefully selected and interpreted. Complete reliance on patch testing is as unwise as complete reliance on animal tests. A use test (preferably coupled with patch tests) has been found useful for predictive testing purposes. SUMMARY It has been shown that animal skin or eye tests are not always reliable indices of irritation potential of finished cosmetic products. Human eye instillation tests should be conducted after rabbit eye tests to ascertain safety. The 48-hour closed patch test appears to be valid if it is negative, i.e., causes no reactions. In this case, the product under test should be a good marketing risk. If the patch test causes irritation, it should be coupled with or followed by a carefully controlled in-use test. It has been shown that the 48-hour closed patch test can be used as a guide in the formulation or improvement of finished cosmetic products. (Received September 25, 1963) * Such a conclusion, incidentally, is a feature of the recently introduced Harris Bill (H.R. 8418).
172 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS REFERENCES (1) R.L. Goldemberg, Proc. Sci. Sect. Toilet Goods ,'lssoc. No. 38, 34 (1962). (2) M.A. Schneiderman, 7. $oc. Cosmetic Chemists, 14, 227 (1963). (3) I.H. Jurow, Food, Drug, Cosmetic Law 7., 18, 97 (1963). (4) H. L. Rubenkoenig and R. A. Quisno, Proc. $ci. Sect. Toilet Goods ,'lssoc., No. 28, 6 (1957). (5) J. H. Draize, in ,'lppraisal of the Safety of Chemicals in Foods, Drugs and Cosmetic,s Assoc. Food and Drug Officials of the U.S., 1959. (6) P. Flesch and S. B. Goldstone, 7. Invest. Dermatol., 18, 267 (1952). (7) R. G. Boughton, etal.,Ibid., 24, 179 (1955). (8) British Patent No. 884,688 Dec. 13, 1961. (9) E.O. Butcher, •.Invest. Dermatol., 16, 85 (1951). (10) K.L. Russell and S. G. Hoch, Proc. $ci. Sect. Toilet Goods ,'lssoc., No. 37, 27 (1962). (11) A. W. McKenzie and R. B. Stoughton, ,'I.M.,'I. ,'lrch. Dermatol., 86, 608 (1962). (12) A.W. McKenzie, Ibid., 86, 611 (1962). (13) E. Cronin and R. B. Stoughton, Ibid., 87, 445 (1962). cf. also, Brit. 7. Dermatol., 74, 265 (1962). (14) A.M. Kligman, Method of Differentiating Mildly Irritating Chemicals, talk presented before the New York Sect. Soc. Cosmetic Chemists April 2, 1963. (15) W. G. Hbckstra and P. H. Phillips, 7-Invest. Dermatol., 40, 79 (1963). (16) R. Klippel, Zentr. allgem. Pathol. u. pathol. ,'lnato., 97, 44 (1957).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)