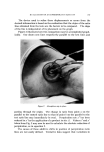
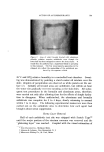
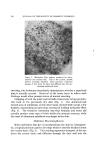
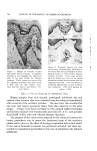
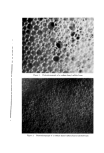
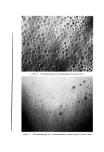
8O6 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Type of Discharge The type of discharge of the products is indicated in the tables. Some of the products had a quiet discharge, while others had a noisy dis- charge. Some samples also discharged as liquids which subsequently expanded into foams. These products are described as having a liquid discharge. Others discharged a mixture of liquid and foam which also expanded into a foam. These are described as having a semiliquid dis- charge. The remaining products gave an immediate foam during dis- charge. Microscopic Examination Observations of the foam structures were made using a Bausch and Lomb Sterozoom microscope, Model BVB-73, equipped with a 10X paired wide field eyepiece and a power pod magnification of 3 X. Ap- proximate bubble sizes in the foams were determined using a microm- eter disc (•31-16-08) which measured intervals of 0.001 in. The fact must be stressed that the bubble sizes reported are approxi- mate. Some of the cells appeared to be fairly round, while others were very irregular in shape. The dimensions given in the report indicate the largest dimension of a specific bubble. All of the foams had bubbles which varied considerably in size. The sizes of the smallest and the largest bubbles were determined in order to show the range of sizes. No attempt was made to determine the frequency of any particular size. Microphotographs were obtained with a Spencer trioeular single- stage microscope, manufactured by the American Optical Company. It was equipped with a 15X eyepiece and a 3.5 objective lens. The earnera was focused through 10X eyepieces with 3.5 objectives. The pictures were taken with a MP-3 Polaroid Multipurpose Industrial View earnera with a 4 X 5 film adapter, using surface illumination and a one second exposure. Preparation of the Aerosol Emulsions The artionic surfactants, sodium lauryl sulfate and the trierhanoi- amine salts of lauric, myristic, palmitie, and stearic acids were used in the present studies. Although sodium lauryl sulfate is not suitable for packaging in metal aerosol containers as a result of its corrosive effect, it has been widely used in complex formation studies in nonaerosol sys- tems and much information was already available about combinations of sodium lauryl sulfate and the long-chain alcohols. The trierhanoi- amine salts of the fatty acids are used extensively in aerosol products,
COMPLEX FORMATION IN AEROSOLS 8O7 and an investigation of systems with these surfactants was particularly desirable from a practical viewpoint. Emulsions containing the triethanolamine soaps were prepared as follows: The fatty acid was heated until it melted (about 60-70 øC). An equimolar quantity of triethanolamine was dissolved in the water, and the aqueous solution was heated to about the same temperature as that of the melted fatty acid. The hot aqueous solution was then added slowly with stirring to the fatty acid. After the addition was complete, the mixture was removed from the heat and allowed to cool to room temperature with stirring. When alcohols were included in the formulation, they were melted together with the fatty acid. Aqueous solutions of sodium lauryl sulfate were prepared by dis- solving the surfactant in water. When alcohols were included, they were melted, and the aqueous solution of sodium lauryl sulfate, heated to the same temperature as that of the alcohols, was added with stirring to the alcohols. Concentrations of the Surfactants The concentrations of the surfactants in the aqueous phase were chosen so that the stability of the emulsions and foams from the sur- factants alone would be low. Thus, any effect of the alcohols upon the system would be noticeable. Sodium lauryl sulfate, triethanolamine laurate, and triethanolamine myristate were normally used at concen- trations of 0.10 m in the aqueous phase. On a weight percent basis, this amounted to about a 3.5% solution for these compounds. The addition of sufficient alcohol to produce a 1:1 molar ratio with the sur- factant increased the weight percent to about 4.5%, depending upon the molecular weight of the alcohol. When other concentrations are used, this is noted in the tables. Triethanolamine palmirate and stearate normally were used at con- centrations of 0.025 m in the aqueous phase. This gave solutions of slightly over 1% on a weight basis. The addition of sufficient alcohol to give a 1:1 molar ratio with the alcohol increased the weight percent of the surfactant-alcohol complex to slightly over 2%. Composition of the Aerosol Emulsions Unless otherwise indicated, all aerosol emulsions were prepared with a composition of 90% aqueous phase and 10% Freon-12/Freon-114* (40/60) propellant. In some cases other propellants were tested, and this is indicated in the tables. * Freon is a registered trademark of E. I. du Pont de Nemours and Co., Wilmington, Del.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)