818 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS sequently expanded into a [oanL However, the rate at which the product expanded into a foam after it had been discharged was a function of the chain length of the alcohol. For example, the discharge from triethanolamine stearate alone expanded almost immediately into a foam, and was followed by that with lauryl alcohol. The discharge Table IX Effect of Various Alcohols upon Properties of Triethanolalnine-Fatty Acid Syste•ns Fatty Acid Alcohol Emulsion Properties Foam Properties Viscosity Stability Stability Drainage Stiffness Laurie Lauryl x xx xx - - x Myristyl x xx xx - - xx Cetyl xx xx xx - - x Stearyl x xx o o o Oleyl o o o - - o Cholesterol x xx - o Myristic Lauryl xx xx x - - o Myristyl xx xx xx - - o Cetyl xx xx xx - - o Stearyl x xx x Oleyl x xx x o Cholesterol xx xx Palmitic Lauryl o o x o o Myristyl xx xx xx - - o Cetyl xx xx xx - - o Stearyl xx xx xx - - o Cholesterol x xx o - Stearic Lauryl o o o o o Myristyl x xx xx - - Cetyl xx xx xx - - o Stearyl xx xx xx - - o Cholesterol xx xx - o - x = Positive effect. xx = Pronounced positive effect. o = Essentially no effect. - -- Negative effect. - - = Pronounced negative effect. from the system containing myristyl alcohol required still more time to expand into a foam, and that with stearyl alcohol required almost five minutes for expansion into a foam. The rate at which the liquid dis- charges expand into a foam may be an indication of the relative strength of the molecular complexes. The strongest complexes might be expected to show the most resistance to expansion during vaporization of the propellant after the product had been discharged. The foam density also increased in the triethanolamine stearate/
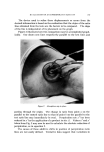
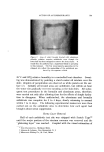
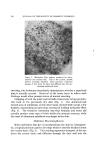
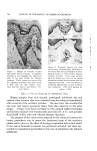
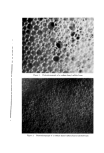
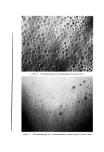
COMPLEX FORMATION IN AEROSOLS 819 alcohol series as the molecular weight of the alcohol increased. Again, this effect may be related to the strength of the molecular complex. Microscopic ]•xamination--Microscopic examination of the tri- ethanolamine laurate and triethanolamine laurate/lauryl alcohol foams did not reveal any marked differences, although the presence of lauryl alcohol appeared to cause a slight decrease in bubble size. However, the addition of cetyl alcohol to triethanolamine palmitate systems or the addition of stearyl alcohol to triethanolamine stearate foams caused a very noticeable decrease in bubble size. This effect of the molecular complexes upon the bubble size of the foams is similar to that noted with the sodium lauryl sulfate foams. The effect of stearyl alcohol in decreasing the bubble size of triethanolamine stearate foams is illustrated by the microphotographs in Figs. 6 and 7. These foams had the com- positions described in Table VII. The effect of cholesterol upon the bubble size of foams was opposite to that observed with the long-chain alcohols. Cholesterol caused a noticeable increase in bubble size. This is illustrated by the micro- photographs in Figs. 8 and 9 of triethanolamine palmirate systems with and without cholesterol. The data in Table VIII also show that cho- lesterol decreased foam stiffness, which again is an effect opposite to that found with long-chain alcohols. These two effects, the increase in bubble size and the decrease in foam stiffness, suggest that the triethanol- amine-fatty acid/cholesterol complex is very fluid, and during discharge the vaporization of the propellant into a gas expands the film more than when cholesterol is not present. This would explain both the increase in bubble size and the decrease in foam stiffness. Variation in Alcohol Concentration The effect of increasing concentrations of alcohol with triethanol- amine laurate/lauryl alcohol and triethanolamine myristate/myristyl alcohol systems is shown in Tables X and XI. In the triethanolamine laurate system, increasing concentrations of lauryl alcohol caused an increase in emulsion stability and a decrease in foam drainage. There was a slight tendency toward increasing foam stiffness. Increasing lauryl alcohol concentration also increased foam stability. The foams with no lauryl alcohol or with a soap/lauryl alcohol ratio of l:•/g wetted paper immediately after discharge. That with a soap/ alcohol ratio of l:lff2 wetted paper in about 25 minutes, and the foam with a soap/alcohol ratio of l:a/g wetted in about an hour. These re- sults correlated essentially with the foam drainage results.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)