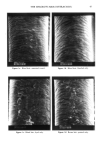
88 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS a) • -------- ' ---•---e --. Prevan Prevan-Bronopol Prevan-Germall 11 5 7 14 21 28 35 Days Bronopol Preyan b) t = 0 t = 5 weeks • Bronopol - -- Prevan interaction product Prevan ------- • Germall ll5 •E' Prevan Germall 11 5 •1 ----------- interaction product Figure 1. a) Trend of DHA.Na concentrations vs. time. b) HPLC runs of binary mixtures DHA.Na- Bronopol © (see method la) and DHA.Na-Germall 115 © (see method lb). with an integral three times each of the two mentioned above. The appearance of the spectrum is very dissimilar to that of DHA (Figure 5): a one-proton singlet at 8 16.60, a one-proton quartet at 8 5.93 with coupling constant of 0.7 Hz, a singlet at 8 2.67, and a doublet at 8 2.29, both with a three-proton integral. Noticeable is the disappear- ance in the spectrum of compound 1 of the acetyl group at 8 2.67, and the mainte- nance of the methyl group and that of the corresponding coupled (J = 0.7 Hz) proton at 8 2.26 and 8 5.88, respectively. The other noticeable feature is the great variation of chemical shift from 8 16.60 to 8 3.29 for the remaining peak. In the first case it can be due to a very deshielded proton, attributable to the acidic hydrogen atom in the 3-posi- tion, while in the second it could originate from a slightly deshielded aliphatic proton(s). According to the molecular weight, the molecule should have a symmetry plane, and therefore the latter peak must be attributable to a methylene moiety. From
REACTION OF DEHYDROACETIC ACID AND FORMALDEHYDE 89 I. 280 1.02o 0.768 0.512 0.256 4 8 min. O. 16 O. 32 O. 48 O. 64 O. 80 Formaldehyde mg/ml Figure 2. Chromatographic pattern of a DHA.Na/formaldehyde water solution five weeks after storage and DHA.Na behavior in dependence on formaldehyde concentration. the above-reported data and from comparison with simple calculations of chemical shift, based on the usual additivity rules, we suggest the structure 3,7-dimethyl- 1H, 9H, 10H-dipyrano[4,3-b: 3 ', 4'-e]pyran- 1,9-dione (7). The 70 eV E1 mass spectrum of compound 1 is shown in Figure 3, while the related 1 OO- 246 Rel. Ab. 80- 60- 40- 20- 149 I 175 69 85 J 161 I ß J,,IL,,i,,,J .... , ..... I,,...,,, ..... ,,, ........ ,,, .... .•.,. ,...•L .... ,,i .... I, I i I 50 100 150 203 217 ,, ,, • ,, I,,i • Ii 200 250 m/z Figure 3. 70 eV EI mass spectrum of compound 1.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)