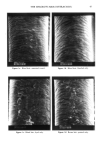
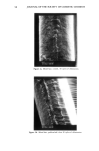
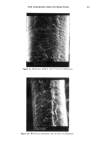
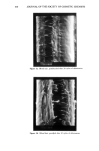
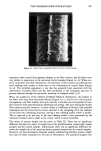
92 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS o o O 2 4 6 min. O 2 4 o o 6 min. o 0 2 4 6 min. Reference sample cosmetic emulsion cosmetic emulsion preserved with Prevan preserved with Prevan and Germall 115 and BronoDol Figure 6. HPLC runs of cosmetic emulsions preserved with DHA.Na-Bronopol © and DHA.Na-Germall 115 © after dilution with THF/H20 (see method 2). molecular weight higher than DHA, due to the reaction of two molecules of DHA with formaldehyde. ACKNOWLEDGMENT This work was supported by a grant from the Italian C.N.R. Special Project on Fine Chemicals. REFERENCES (1) A. Bettero, A. Semenzato, G. Aversa, and C. A. Benassi, Imidazolidinilurea e formaldeide nei pro- dotti cosmetici, Chimica Oggi, 11, 29-32 (1985). (2) A. Bettero, F. Galiano, S. Daolio, and C. A. Benassi, The characterization of isothiazolinone pre- servatives in cosmetics, J. Pharm. Biotaed. Anal., 3, 581-587 (1985). (3) A. Bettero, A. Semenzato, and C. A. Benassi, Preservatives in cosmetics. Developments on direct characterization, Proc. 14 •h IFSCC, Barcelona, 16-19 September 1986, Vol. I, 187-194. (4) C. A. Benassi, A. Semenzato, M. Lucchiari, and A. Bettero, Dehydroacetic acid sodium salt stability in cosmetic preservative mixtures. Int. J. Cosmet. Sci. 1988 (in press). (5) C. A. Benassi, A. Bettero, A. Semenzato, and R. Cerini, Correlazione tra analisi chimica e previ- sione di stabillth microbiologica di un prodotto cosinerico. Proc. VII Congresso Naz. Div. Chim. Farm. Soc. Chim. It., Pisa, 22-26 Settembre 1987. (6) A. P. Bruins, K. R. Jenning, and S. Evans, The observation of metastable transitions in a double-fo- cussing mass spectrometer using a linked scan of the electric sector and magnetic sector fields, Int. J. Mass Spectrom. Ion Phys., 26, 395-404 (1978). (7) M. Moreno-Mafias, J. Ribas, and A. Virgili: The 3,6,9-trioxanthracene and 3,6-dioxa-9-thianthra- cene ring systems by cyclization of 1,1-bis[4-hydroxy-6-methyl-2-oxo-2H-pyran-3yl] alkanes, Syn- thesis, 3, 699-701 (1985). (8) Q. N. Porter, Mass Spectrometry of Heterocyclic Compounds (Wiley, New York, 1985), pp. 198-211. (9) A. Bettero, B. Casetta, F. Galiano, E. Ragazzi, and C. A. Benassi, Rheological and spectroscopic behaviour of cosmetic products. Application to sulphur analysis. Fresenius Z. Anal. Chem., 318, 525-528 (1984).
J. Soc. Cosmet. Chem., 39, 93-105 (March/April 1988) The chlorine-hair interaction. III. Effect of combining chlorination with cosmetic treatments on hair properties N. B. FAIR and B. S. GUPTA, University of Missouri-Columbia, Columbia, MO 65211 (N.B.F.), and North Carolina State University, Raleigh, NC 27695-8301 (B.S.G.) Received April 23, 1987. Presented in part at the Annual Scientific Meeting of the Society of Cosmetic Chemists, New York, December 1987. Synopsis The results of a study of the combined effect of chlorination and a cosmetic treatment on selected physical properties of human hair fibers are presented. The hair was either bleached, dyed, or permed as a pretreat- ment (before the chlorination procedure) or as a posttreatment (after the chlorination procedure). Cosmetic treatments given as pretreatments did not affect frictional properties and surface morphology as markedly as did the cosmetic treatments given as posttreatments. Bleaching and dyeing produced more pronounced effects on surface properties and weight loss of the hair fibers, while perming had the greater effect on tensile properties. INTRODUCTION In a previous paper (1), we described the results of a study of the effects of chlorine on various hair properties. It is clear, however, that the history of a given human hair may involve many more treatments than chlorination, but little information is available in the published literature on the effects of combining treatments with chlorination on the physical properties of this fiber. This paper reports the results of a study in which each of the treatments of hydrogen peroxide bleaching, oxidative dyeing, and permanent waving were combined with different durations of exposure to dilute concentrations of chlorine. MATERIALS AND METHODS SAMPLE PREPARATION/CHLORINATION PROCEDURE Natural blond and dark brown Caucasian hair purchased from De Meo Brothers Com- pany were used in this study, with samples prepared to suit the physical property being studied (1). Solutions with chlorine concentrations of 10 ppm were prepared by dilution of a sodium hypochlorite solution with deionized water. Two chlorination procedures were used, one for the hair mounted on frames for friction, morphological, and knot strength tests, and the other for hair wound into loops or mounted onto tabs for weight loss or tensile tests, respectively. Treatments were carried 93
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)